��Ŀ����
18�� ij�о�С�������һ���µ����룺����ԭ���ԭ�������һ��H2��Cl2ȼ�ϵ�أ�ԭ����ܷ�ӦΪ��H2+Cl2=2HCl���ڶ����ṩ���ܵ�ͬʱ���ɵõ���ҵ�Ͼ�����Ҫ��;�Ľ�Ũ�����ᣮ������Ƶ�ԭ���װ�����£����������缫��Ϊ��ײ��缫�������й���������ȷ���ǣ�������
ij�о�С�������һ���µ����룺����ԭ���ԭ�������һ��H2��Cl2ȼ�ϵ�أ�ԭ����ܷ�ӦΪ��H2+Cl2=2HCl���ڶ����ṩ���ܵ�ͬʱ���ɵõ���ҵ�Ͼ�����Ҫ��;�Ľ�Ũ�����ᣮ������Ƶ�ԭ���װ�����£����������缫��Ϊ��ײ��缫�������й���������ȷ���ǣ�������| A�� | aΪ������ͨ�������Ϊ���� | |
| B�� | �����ĵ缫��ӦʽΪ��Cl2+2e-=2Cl- | |
| C�� | ������b�������������·��a�� | |
| D�� | ԭ����ڲ���H+������Cl-�������� |
���� ԭ����ܷ�ӦΪ��H2+Cl2=2HCl���ݵ��������֪��aΪ�����������ڸ���ʧ�������������ӣ�bΪ�����������������õ������������ӣ�����������b����a��ԭ����������������������������������ݴ˷�����
��� �⣺ԭ����ܷ�ӦΪ��H2+Cl2=2HCl���ݵ��������֪��aΪ�����������ڸ���ʧ�������������ӣ�bΪ�����������������õ������������ӣ�����������b����a��ԭ���������������������������������
A���ݵ��������֪��aΪ�����������ڸ���ʧ�������������ӣ���A��ȷ��
B��bΪ�����������������õ������������ӣ��缫��ӦʽΪCl2+2e-=2Cl-����B��ȷ��
C������������b����a����C��ȷ��
D��ԭ�����������������������������������D����
��ѡD��
���� ���⿼����ԭ��صĵ缫��Ӧ�Լ���������͵���������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
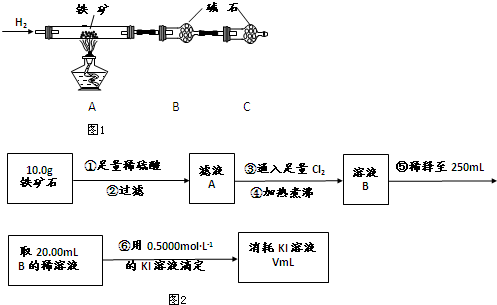
8��ijѧ��С�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
�ٽ���ʽ�ζ���������ˮϴ�����ñ����ᣨ0.1000mol/L����Һ��ϴ2�飬ע������ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0����0�����¿̶ȣ�����ƿ������ˮϴ����Ҳ�ø�������Һ��ϴ2�飻����ʽ�ζ����зų�20.00mL��Һ����ƿ�У�
�ڽ���ʽ�ζ���������ˮϴ�����ô���NaOH��Һ��ϴ2�飬���ڵζ��ܼ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0����0�����¿̶ȣ�
������ƿ�е����̪��ָʾ�������еζ�����pH�Ƽ�¼pH�仯���ζ���ָʾ���պñ�ɫ���Ҳ������ϻָ�ԭ����ɫΪֹ����¼��ʽ�ζ���Һ��������ټ����ζ�������¼pH�������Ա仯Ϊֹ��
�ظ�����ȡ�����ζ�����2�Σ���¼ÿ��ʵ�����ü���Һ�����
�ش��������⣺
��1��ͼ1����2���ζ��ܣ�����b��ѡ�a������b��������ʽ�ζ��ܣ�
��2���ζ������У��ߵα�ҡ����ƿ���۾�Ӧ�۲�a��ѡ�a������b������
a����ƿ����Һ��ɫ�ı仯 ��b���ζ�����Һ��ı仯
��3����С���ڲ��������һ�����ԵĴ���������ɴ���ɲⶨ���ƫ�� ��ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��4��ͼ2�иõζ����̵ĵζ����������е�a��ѡ�a������b������

��5���й����ݼ�¼���£�
���С�����õ�NaOH��Һ�����ʵ���Ũ��Ϊ0.10mol/L��
�ٽ���ʽ�ζ���������ˮϴ�����ñ����ᣨ0.1000mol/L����Һ��ϴ2�飬ע������ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0����0�����¿̶ȣ�����ƿ������ˮϴ����Ҳ�ø�������Һ��ϴ2�飻����ʽ�ζ����зų�20.00mL��Һ����ƿ�У�
�ڽ���ʽ�ζ���������ˮϴ�����ô���NaOH��Һ��ϴ2�飬���ڵζ��ܼ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0����0�����¿̶ȣ�
������ƿ�е����̪��ָʾ�������еζ�����pH�Ƽ�¼pH�仯���ζ���ָʾ���պñ�ɫ���Ҳ������ϻָ�ԭ����ɫΪֹ����¼��ʽ�ζ���Һ��������ټ����ζ�������¼pH�������Ա仯Ϊֹ��
�ظ�����ȡ�����ζ�����2�Σ���¼ÿ��ʵ�����ü���Һ�����
�ش��������⣺
��1��ͼ1����2���ζ��ܣ�����b��ѡ�a������b��������ʽ�ζ��ܣ�
��2���ζ������У��ߵα�ҡ����ƿ���۾�Ӧ�۲�a��ѡ�a������b������
a����ƿ����Һ��ɫ�ı仯 ��b���ζ�����Һ��ı仯
��3����С���ڲ��������һ�����ԵĴ���������ɴ���ɲⶨ���ƫ�� ��ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��4��ͼ2�иõζ����̵ĵζ����������е�a��ѡ�a������b������

��5���й����ݼ�¼���£�
| ʵ����� | �����Һ�����mL�� | �������������Ʊ�Һ�������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.50 | 20.70 |
| 2 | 20.00 | 2.00 | 22.00 |
| 3 | 20.00 | 1.00 | 20.80 |
9��Na2S2O3•5H2O���׳ƺ�����������ҵ���õ�һ�ֶ�Ӱ������ҵ���Ƶõ�Na2S2O3•5H2O�����п��ܺ���������Na2SO3��Na2SO4���ʣ�Ϊ�˲ⶨij������Ʒ�ijɷ֣���ȡ����������ͬ�ĸ���Ʒ���ֱ������ͬŨ�ȵ�������Һ30mL����ַ�Ӧ���˳�������Һʹ���ɵ�SO2ȫ���ݳ�����Na2S2O3+H2SO4��Na2SO4+SO2��+S��+H2O��
����й�ʵ���������£���״������
��Ħ��������Na2S2O3•5H2O 248g/mol�� Na2SO3126g/mol��Na2SO4 142g/mol��
��1����������������Һ�����ʵ���Ũ�ȣ�
��2����������ʵ�����ݣ�����Ʒ��d����дѡ����ĸ����
A��������Na2S2O3•5H2O
B�����к�Na2S2O3•5H2O��Na2SO3����Na2SO4
C������Na2S2O3•5H2O��Na2SO3��Na2SO4
��3������30.16g����Ʒ��һ����������������Һ����ȣ������ۣ�����������������aL���ڲ�ͬȡֵ��Χʱ�����ɵ�S02�����b L����ֵ�����ú�a�Ĺ�ϵʽ��ʾ����
����й�ʵ���������£���״������
| ��һ�� | �ڶ��� | ������ | |
| ��Ʒ������/g | 7.54 | 15.08 | 35.00 |
| ������������/L | 0.672 | 1.344 | 2.688 |
| �������/g | 0.8 | 1.6 | 3.2 |
��1����������������Һ�����ʵ���Ũ�ȣ�
��2����������ʵ�����ݣ�����Ʒ��d����дѡ����ĸ����
A��������Na2S2O3•5H2O
B�����к�Na2S2O3•5H2O��Na2SO3����Na2SO4
C������Na2S2O3•5H2O��Na2SO3��Na2SO4
��3������30.16g����Ʒ��һ����������������Һ����ȣ������ۣ�����������������aL���ڲ�ͬȡֵ��Χʱ�����ɵ�S02�����b L����ֵ�����ú�a�Ĺ�ϵʽ��ʾ����
6������˵����ȷ���ǣ�������
| A�� | ���������Ǵ������ܼӿ췴Ӧ���� | |
| B�� | �¶�Խ�ߣ������Ĵ�Ч��Խ�� | |
| C�� | ʳƷ��װ���еĿ����������ǡ�������������ʹ������ԭ��Ӧ�����ʼ��� | |
| D�� | �����¶ȣ����ȷ�Ӧ�ͷ��ȷ�Ӧ�ķ�Ӧ���ʶ��ӿ� |
13����NA��ʾ����٤��������ֵ������˵����ȷ���ǣ�������
| A�� | �ڸ��¸�ѹ�£�28 gN2��6g H2��ַ�Ӧ����NH3�ķ�����Ϊ2NA | |
| B�� | 1mol/LFeCl3 ��Һ������Fe3+����ĿС��NA | |
| C�� | �����£�1L pH=12��Ba��OH��2 ��Һ�к�OH-����ĿΪ2NA | |
| D�� | 23g NO2��N2O4�Ļ�������к��е�ԭ����Ϊ0.5NA |
8�����ֶ�����Ԫ�ص�ԭ�Ӱ뾶��ijЩ���ϼۼ��±��������ж�����˵����ȷ���ǣ�������
| Ԫ�ش��� | A | B | D | E | G | H | I | J |
| ���ϼ� | -1 | -2 | +4��-4 | -1 | +5��-3 | +3 | +2 | +1 |
| ԭ�Ӱ뾶/nm | 0.071 | 0.074 | 0.077 | 0.099 | 0.110 | 0.143 | 0.160 | 0.186 |
| A�� | I��DB2��ȼ���������ֻ����� | |
| B�� | B��E��J�����Ӱ뾶�ɴ�С˳����E��J��B | |
| C�� | GԪ�صĵ��ʲ�����ͬ�������� | |
| D�� | B��J���γɼȺ����Ӽ��ֺ����ۼ��Ļ����� |

 ��I
��I ��
�� ��
�� ��
�� ��ͬ���칹�����࣬�������ڷӵ�ͬ���칹�干��9�֣���Щͬ���칹���У��ں˴Ź������������ĸ����շ��ͬ���칹��Ľṹ��ʽΪ��
��ͬ���칹�����࣬�������ڷӵ�ͬ���칹�干��9�֣���Щͬ���칹���У��ں˴Ź������������ĸ����շ��ͬ���칹��Ľṹ��ʽΪ�� ��
��