��Ŀ����
��1�������й��к͵ζ��IJ�����
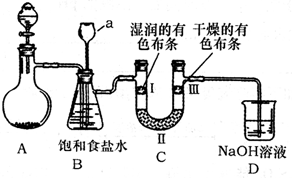
���ñ�Һ��ϴ�ζ��ܣ������ζ�����ע�����Һ���ۼ��ζ����Ƿ�©ˮ���ܵζ����ݵμ�ָʾ���ڴ���Һ����ϴ�ӣ�
��ȷ�IJ���˳����
��2�����ڷ�Ӧ2L��g��+3M��g��?xQ��g��+3R��g�������ݻ�Ϊ2L���ܱ������У���2mol����L��3mol����M��ϣ�����Ӧ��2min���ƽ��ʱ����2.4mol����R����0.8mol ����Q����x��ֵΪ
���ñ�Һ��ϴ�ζ��ܣ������ζ�����ע�����Һ���ۼ��ζ����Ƿ�©ˮ���ܵζ����ݵμ�ָʾ���ڴ���Һ����ϴ�ӣ�
��ȷ�IJ���˳����
�ۢޢ٢ڢݢ�
�ۢޢ٢ڢݢ�
��2�����ڷ�Ӧ2L��g��+3M��g��?xQ��g��+3R��g�������ݻ�Ϊ2L���ܱ������У���2mol����L��3mol����M��ϣ�����Ӧ��2min���ƽ��ʱ����2.4mol����R����0.8mol ����Q����x��ֵΪ
1
1
��ƽ��ʱM��Ũ����0.3mol/L
0.3mol/L
��L��ת����Ϊ80%
80%
��VR=0.6mol/��Lmin��
0.6mol/��Lmin��
����������1���к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ��Ȳ�����
��3�����ݻ�ѧƽ�������ʽ������з�����������и�����ʽ�����жϣ�
��3�����ݻ�ѧƽ�������ʽ������з�����������и�����ʽ�����жϣ�
����⣺��1���к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ���˳��������ʴ�Ϊ���ۢޢ٢ڢݢ�
��2����2min���ƽ��ʱ����2.4mol����R��0.8mol ����Q��
2L��g��+3M��g��?xQ��g��+3R��g����
��ʼ����mol�� 2 3 0 0
�仯����mol�� 1.6 2.4 0.8 2.4
ƽ������mol�� 0.4 0.6 0.8 2.4
������ʽ�������㣺
����Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȿ�֪��x=1��
ƽ��ʱM��Ũ����
=0.3mol/L��
L��ת����Ϊ
��100%=80%��
2min��R�ķ�Ӧ����
=0.6mol/��L?min����
�ʴ�Ϊ��1�� 0.3 mol/L�� 80%�� 0.6mol/��L min����
��2����2min���ƽ��ʱ����2.4mol����R��0.8mol ����Q��
2L��g��+3M��g��?xQ��g��+3R��g����
��ʼ����mol�� 2 3 0 0
�仯����mol�� 1.6 2.4 0.8 2.4
ƽ������mol�� 0.4 0.6 0.8 2.4
������ʽ�������㣺
����Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȿ�֪��x=1��
ƽ��ʱM��Ũ����
| 0.6mol |
| 2L |
L��ת����Ϊ
| 1.6 mol |
| 2mol |
2min��R�ķ�Ӧ����
| ||
| 2min |
�ʴ�Ϊ��1�� 0.3 mol/L�� 80%�� 0.6mol/��L min����
���������⿼���˻�ѧƽ�������ʽ���㡢ת���ʡ���Ӧ���ʻ����㣬�ѶȲ����ݸ������ɣ�

��ϰ��ϵ�д�
�����Ŀ
��1�������й�ʵ���˵������������۲���ȷ���� ������ţ�
| A���Ʊ�Fe(OH)3����ʱ��Ӧ����ˮ�еμӱ���FeCl3��Һ�����������ȵ���Һ�ʺ��ɫΪֹ�� |
| B���ⶨ�к���ʵ���У�ÿ��ʵ���Ӧ���������¶ȣ���������ʼ�¶ȣ�NaOH��Һ����ʼ�¶Ⱥͷ�Ӧ����Һ������¶ȡ� |
| C����һ�����ʵ�����Һ���ƵĹ����У�û��ϴ���ձ��Ͳ�����������ʱ��ˮ�����˿̶��ߡ�����ƿû�и������ʹ������ҺŨ��ƫ�͡� |
| D����FeCl3��Һ�е���KI��������Һ����Һ����ɫ�� |
F����֤AgNO3��Һ���Ƿ����Al(NO3)3���ɼ��������ˮ�����а�ɫ�����������Al(NO3)3
G��ϡ��Ũ����ʱ��Ҫ��ˮ�������ڻ���ע��Ũ�����У����ò��������Ͻ��衣
��2��ʵ��������500ml 0.1mol/L��Na2CO3��Һ������������ƽ��ȡ̼���Ʒ�ĩ g��
����ʱӦѡ�õ������У�������ƽ��ҩ�ס��ձ�����Ͳ�� ��
 ��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᣮ
��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᣮ