��Ŀ����
ƽ��ϲ�dz��õ��к�θ���ҩ����Ļ�ѧ�ɷ��ǿ��Ա�ʾΪ��MgxAly��OH��zCO3?nH2O��������Ԫ�ص���������Ϊ0.040��ȡ�ü�ʽ��30.1g��������ȫ�ֽ�������״����1.12L���壮����2.0mol?L-1����450mLʹ��������ȫ�ܽ⣮����������ʽ��������������Һ�м���������������ƣ����ˣ���������и������17.4g���������У�
��1��̼��������ʵ�����
��2��þ���ӵ����ʵ�����
��3�����������ӵ����ʵ�����
��4����ʽ̼���εĻ�ѧʽ��
�⣺��1��ȡ�ü�ʽ��30.1g��������ȫ�ֽ�������״����1.12L����Ϊ������̼�����ʵ���Ϊ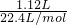 =0.05mol������̼Ԫ���غ��֪n��CO32-��=n��CO2��=0.05mol��
=0.05mol������̼Ԫ���غ��֪n��CO32-��=n��CO2��=0.05mol��
��̼��������ʵ���Ϊ0.05mol��
��2���ü�ʽ��30.1g��������������Һ�м���������������ƣ����ˣ���������и������17.4g��Ϊ������þ��������������þ�����ʵ���Ϊ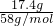 =0.3mol������þԪ���غ��֪n��Mg2+��=n[Mg��OH��2]=0.3mol����þ���ӵ����ʵ���Ϊ0.3mol��
=0.3mol������þԪ���غ��֪n��Mg2+��=n[Mg��OH��2]=0.3mol����þ���ӵ����ʵ���Ϊ0.3mol��
��3���ü�ʽ��30.1g������2.0mol?L-1����450mLʹ��������ȫ�ܽ⣬��Ӧ����Һ������Ϊ�Ȼ�þ���Ȼ��������ݵ���غ��֪n��Cl-��=n��OH-��+2n��CO32-������n��OH-��=n��Cl-��-2n��CO32-��=0.45L��2mol/L-2��0.05mol=0.8mol���������������ʵ���Ϊ0.8mol��
��4��̼��������ʵ���Ϊ0.05mol����30.1g�ü�ʽ�ε����ʵ���Ϊ0.05mol��
����þ���ӵ����ʵ���Ϊ0.3mol����0.05mol��x=0.3mol����x=6��
�ɵ���غ��֪n��Cl-��=2n��Mg2+��+3n��Al3+������n��Al3+��= ��0.45L��2mol/L-0.3mol��2��=0.1mol������0.05mol��y=0.1mol����y=2��
��0.45L��2mol/L-0.3mol��2��=0.1mol������0.05mol��y=0.1mol����y=2��
����������Ϊ0.8mol��0.05mol��z=0.8mol����z=16��
�ü�ʽ�ε���Է�������Ϊ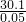 =602����24��6+27��2+17��16+60+18n=602�����n=4��
=602����24��6+27��2+17��16+60+18n=602�����n=4��
�ʸü�ʽ�εĻ�ѧʽΪ��Mg6Al2��OH��16CO3?4H2O��
�𣺸ü�ʽ̼���εĻ�ѧʽΪMg6Al2��OH��16CO3?4H2O��
��������1��ȡ�ü�ʽ��30.1g��������ȫ�ֽ�������״����1.12L����Ϊ������̼������n= ���������̼�����ʵ���������̼Ԫ���غ����̼��������ʵ�����
���������̼�����ʵ���������̼Ԫ���غ����̼��������ʵ�����
��2���ü�ʽ��30.1g��������������Һ�м���������������ƣ����ˣ���������и������17.4g��Ϊ������þ������������n= ����������þ�����ʵ���������þԪ���غ����þ���ӵ����ʵ�����
����������þ�����ʵ���������þԪ���غ����þ���ӵ����ʵ�����
��3���ü�ʽ��30.1g������2.0mol?L-1����450mLʹ��������ȫ�ܽ⣬��Ӧ����Һ������Ϊ�Ȼ�þ���Ȼ��������ݵ���غ��֪n��Cl-��=n��OH-��+2n��CO32-�����ݴ˼��㣻
��4������̼���ȷ��30.1g�ü�ʽ�ε����ʵ���������þ���ӵ����ʵ���ȷ��x��ֵ���ɵ���غ��֪n��Cl-��=2n��Mg+��+3n��Al3+�����ݴ˼���n��Al3+����ȷ��y�����������������������ʵ���ȷ��z��ֵ�������øü�ʽ�ε���Է�������ȷ��n��ֵ��
���������⿼�黯ѧʽ��ȷ�������ʵ������йؼ���ȣ��Ѷ��еȣ�ע�������غ���еļ��㣮
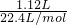 =0.05mol������̼Ԫ���غ��֪n��CO32-��=n��CO2��=0.05mol��
=0.05mol������̼Ԫ���غ��֪n��CO32-��=n��CO2��=0.05mol����̼��������ʵ���Ϊ0.05mol��
��2���ü�ʽ��30.1g��������������Һ�м���������������ƣ����ˣ���������и������17.4g��Ϊ������þ��������������þ�����ʵ���Ϊ
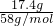 =0.3mol������þԪ���غ��֪n��Mg2+��=n[Mg��OH��2]=0.3mol����þ���ӵ����ʵ���Ϊ0.3mol��
=0.3mol������þԪ���غ��֪n��Mg2+��=n[Mg��OH��2]=0.3mol����þ���ӵ����ʵ���Ϊ0.3mol����3���ü�ʽ��30.1g������2.0mol?L-1����450mLʹ��������ȫ�ܽ⣬��Ӧ����Һ������Ϊ�Ȼ�þ���Ȼ��������ݵ���غ��֪n��Cl-��=n��OH-��+2n��CO32-������n��OH-��=n��Cl-��-2n��CO32-��=0.45L��2mol/L-2��0.05mol=0.8mol���������������ʵ���Ϊ0.8mol��
��4��̼��������ʵ���Ϊ0.05mol����30.1g�ü�ʽ�ε����ʵ���Ϊ0.05mol��
����þ���ӵ����ʵ���Ϊ0.3mol����0.05mol��x=0.3mol����x=6��
�ɵ���غ��֪n��Cl-��=2n��Mg2+��+3n��Al3+������n��Al3+��=
 ��0.45L��2mol/L-0.3mol��2��=0.1mol������0.05mol��y=0.1mol����y=2��
��0.45L��2mol/L-0.3mol��2��=0.1mol������0.05mol��y=0.1mol����y=2������������Ϊ0.8mol��0.05mol��z=0.8mol����z=16��
�ü�ʽ�ε���Է�������Ϊ
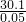 =602����24��6+27��2+17��16+60+18n=602�����n=4��
=602����24��6+27��2+17��16+60+18n=602�����n=4���ʸü�ʽ�εĻ�ѧʽΪ��Mg6Al2��OH��16CO3?4H2O��
�𣺸ü�ʽ̼���εĻ�ѧʽΪMg6Al2��OH��16CO3?4H2O��
��������1��ȡ�ü�ʽ��30.1g��������ȫ�ֽ�������״����1.12L����Ϊ������̼������n=
 ���������̼�����ʵ���������̼Ԫ���غ����̼��������ʵ�����
���������̼�����ʵ���������̼Ԫ���غ����̼��������ʵ�������2���ü�ʽ��30.1g��������������Һ�м���������������ƣ����ˣ���������и������17.4g��Ϊ������þ������������n=
 ����������þ�����ʵ���������þԪ���غ����þ���ӵ����ʵ�����
����������þ�����ʵ���������þԪ���غ����þ���ӵ����ʵ�������3���ü�ʽ��30.1g������2.0mol?L-1����450mLʹ��������ȫ�ܽ⣬��Ӧ����Һ������Ϊ�Ȼ�þ���Ȼ��������ݵ���غ��֪n��Cl-��=n��OH-��+2n��CO32-�����ݴ˼��㣻
��4������̼���ȷ��30.1g�ü�ʽ�ε����ʵ���������þ���ӵ����ʵ���ȷ��x��ֵ���ɵ���غ��֪n��Cl-��=2n��Mg+��+3n��Al3+�����ݴ˼���n��Al3+����ȷ��y�����������������������ʵ���ȷ��z��ֵ�������øü�ʽ�ε���Է�������ȷ��n��ֵ��
���������⿼�黯ѧʽ��ȷ�������ʵ������йؼ���ȣ��Ѷ��еȣ�ע�������غ���еļ��㣮

��ϰ��ϵ�д�
�����Ŀ