��Ŀ����
�����仯����Ӧ�ù㷺��
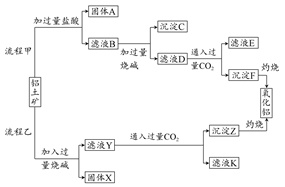
��1�����Ȼ�����һ��ˮ����������ҵ�Ʊ���ˮ���Ȼ�������IJ�����������ͼ��
�ټ��鸱��Ʒ�к���Xʱ��ѡ�õ��Լ��� (�����и��������)��
a��NaOH��Һ b��KSCN��Һ c������KMnO4��Һ d������
�����������У����ɸ���ƷFeCl�������ӷ���ʽΪ
��2���������(K2FeO4)Ҳ��һ��������ˮ����������ҵ�ϣ������������������KOH��Һ�Ʊ�������ء��������У������ĵ缫��ӦʽΪ �����һ��ʱ�����������������28 g�����ڴ˹����У����������������ڱ�״���µ����Ϊ L��
��3�������������ڹ�ҵ��ˮ�Ĵ�����
������Ϊ���ܷ���������������Cd2+�Ĺ�ҵ��ˮ? (��ܡ���)������ݳ����ܽ�ƽ���ԭ��������Ĺ۵�(�ñ�Ҫ�����ֺ����ӷ���ʽ˵��)�� (��֪��25��ʱ���ܶȻ�����Ksp(FeS)=6��310-18��Ksp(CdS)=3��610-29)
�ڹ�ҵ�ϴ�����Cd2+��ˮ�����Բ��ü�̼���Ƶķ�������Ӧ���£�2Cd2++2CO32-+H2O=Cd2(OH)2CO3 +A����A�Ļ�ѧʽΪ ��
+A����A�Ļ�ѧʽΪ ��
��17�֣�
��1����5�֣���c��2�֣���2Fe2++Cl2=2Fe3++2Cl?��3�֣�
��2����5�֣�Fe��6e?+8OH?=FeO42?+4H2O��3�֣�33.6��2�֣�
��3����7�֣����ܣ�1�֣�CdS��FeS�����ܣ��ɷ�������ת��Cd2+(aq)+FeS(s)=CdS(s)+Fe2+(aq)��3�֣�
��CO2��3�֣�
���������������1�������ռ�X������������δ��Ӧ��Cl2������FeCl3������XΪFeCl2������FeCl3��Һ�к���FeCl2���Լ�������KMnO4��Һ����c����ȷ��
��Fe������ʧȥ��������FeO42?����KOH��Һ���������Һ��OH?�μӷ�Ӧ�����������缫����ʽΪ��Fe��6e?+8OH?=FeO42?+4H2O��������������ʧ����������ȣ����Զ�Ӧ��ϵΪ��Fe ~ 6e? ~ 3H2����n(H2)=3n(Fe)=3��28g��56g/mol=1.5mol����״���µ����Ϊ33.6L��
��3������ΪKsp(CdS)С��Ksp(FeS)������CdS��FeS�����ܣ��ɷ�������ת��Cd2+(aq)+FeS(s)=CdS(s)+Fe2+(aq)���������������������Cd2+�Ĺ�ҵ��ˮ��
�ڸ���Ԫ���غ㣬��֪AΪCO2��
���㣺���⿼�黯ѧ�������̵ķ��������ԭ�������㡢���ӷ���ʽ����д�������ܽ�ƽ�⡣

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д��������Ũ�������ܷ����ۻ���ij��ȤС���ͬѧ���ֽ�һ����������Ũ�������ʱ���۲쵽����ȫ�ܽ⣬�������������塣ʵ�������������Լ���0.01 mol/L����KMnO4��Һ��0.1 mol/L KI��Һ��3%H2O2��Һ��������Һ������ˮ������Э������̽��������Һ������ijɷ֡�
��������롿
��.������Һ�еĽ������ӿ��ܺ���Fe2����Fe3���е�һ�ֻ����֣�
��.���������п��ܺ���________�е�һ�ֻ����֡�
| | ʵ����� | Ԥ������ | ���� |
| ��֤����� | ����٣�ȡ����0.01 mol/L����KMnO4��Һ������������Һ | | |
| ����ڣ�________ | | ����Fe3 | |
| ��֤����� | ����������ͨ������װ�� | | ������������ |
��ʵ��̽����

���������ۡ�
��ͬѧ�����������ѡ��KSCN��Һ���������KSCN��H2O2������Һ������ɲ���������̽�����жϸ÷����Ƿ���ȷ���������ۣ�_____________________________