��Ŀ����
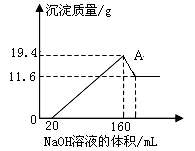
���ᷢ��������ԭ��Ӧ��ʱ��һ������Խϡ����Ӧ�Ļ�ԭ�����е��Ļ��ϼ�Խ�͡�����һ�������������Ͻ���һ����ϡHNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4 mol/L NaOH��Һ������NaOH��Һ�����(V)��������������ʵ���(n)��ϵ��ͼ��ʾ������˵���в���ȷ����


| A�����������C����ֵ |
| B���Ͻ������ᷴӦʱ������� |
| C���Ͻ����������ʵ���Ϊ0.008 mol |
| D���Ͻ��н��������ʵ�����Ϊ0.032 mol |
A
�����������Ӧʼ��û���������ɣ����Եó������е������������ɡ�����Ϊ�����Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ������Ʋ�NԪ����+5�����-3�ۡ���ͼ�ɵ������������������������ҺӦ�������ᷴӦ�������ɳ�������������ȫ����ͼ֪������������������Һ�����������䣬�ɵ���NH4+�����˷�Ӧ����������Ļ�ԭ����Ϊ����李�����Al��OH��3 +OH����AlO2��+2H2O���ó�Al��OH��3�����ʵ���Ϊ����36��34����10��3L��4mol/L��0.008 mol������NH4++OH���TNH3?H2O �ó�n��NH4+������34��31����10��3L��4mol/L��0.012 mol������������ԭ��Ӧ��NԪ����+5�۱�Ϊ-3�ۣ�����������0�۱�Ϊ+3�ۣ��������õ����غ�ó����������ʵ���Ϊ��0.012mol��8����3��0.032mol���μ�NaOH���Ϊ31mlʱ��������ӦΪ��H++OH����H2O����Fe3++3OH����Fe��OH��3����Al3++3OH����Al��OH��3�����ɼ���ó�C������������Һ�����Ϊ0.031L����0.032mol��3����4mol/L��0.007L��7ml������ѡ��A����ȷ������ѡ�����ȷ�ģ���ѡA��
�������������е��Ѷȵ����⣬�����ۺ���ǿ�������߿��������Խ���������ķ�ӦΪ���壬�ص㿼�����ӷ���ʽ����д��������ԭ��Ӧ����ѧ���㡢ͼ�������֪ʶ�㣬���������ǿ��ע�ش��������ԣ�����������ѧ���������������ʹ���˼ά������������ؼ�����ȷ��Ӧ��ԭ�����жϳ�����Ļ�ԭ�����Լ���ʧ����ת���غ�����õȡ�

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д� ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ