��Ŀ����
����ʱ����20mL 0.1mol/L�Ĵ�����Һ�в��ϵ���0.1mol/L��NaOH��Һ����Һ��pH�仯������ͼ��ʾ���ڵζ������У�������Һ������Ũ�ȴ�С��ϵ��������ȷ���ǣ�������
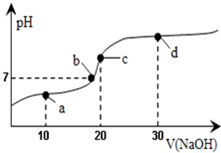
| A��a��ʱ��c��CH3COOH����c��CH3COO-����c��H+����c��Na+����c��OH-�� |
| B��b��ʱ��c��Na+��=c��CH3COO-�� |
| C��c��ʱ��c��H+��=c��CH3COOH��+c��OH-�� |
| D��d��ʱ��c��Na+����c��OH-����c��CH3COO-����c��H+�� |

A��a��ʱ�����������ҺΪCH3COOH��CH3COONa�Ļ�����Һ�����ԣ�Ӧ����c��CH3COO-����c��CH3COOH������A����
B��������Һ����غ��֪��Һ��Ӧ����c��Na+��+c��H+��=c��CH3COO-��+c��OH-������Һ�����ԣ�Ӧ��c��H+��=c��OH-������c��Na+��=c��CH3COO-������B��ȷ��
C��c��ʱ����Һ�ʼ��ԣ�Ӧ��c��H+����c��OH-������C����
D��d��ΪNaOH��CH3COONa�Ļ�����Һ�ʼ��ԣ�����CH3COO-��������ˮ�⣬����c��Na+����c��OH-����c��CH3COO-����c��H+������D��ȷ��
��ѡBD��
B��������Һ����غ��֪��Һ��Ӧ����c��Na+��+c��H+��=c��CH3COO-��+c��OH-������Һ�����ԣ�Ӧ��c��H+��=c��OH-������c��Na+��=c��CH3COO-������B��ȷ��
C��c��ʱ����Һ�ʼ��ԣ�Ӧ��c��H+����c��OH-������C����
D��d��ΪNaOH��CH3COONa�Ļ�����Һ�ʼ��ԣ�����CH3COO-��������ˮ�⣬����c��Na+����c��OH-����c��CH3COO-����c��H+������D��ȷ��
��ѡBD��

��ϰ��ϵ�д�
�����Ŀ
 ��2012?�ֶ�������ģ������ʱ����20mL 0.1mol/L�Ĵ�����Һ�в��ϵ���0.1mol/L��NaOH��Һ����Һ��pH�仯������ͼ��ʾ���ڵζ������У�������Һ������Ũ�ȴ�С��ϵ��������ȷ���ǣ�������
��2012?�ֶ�������ģ������ʱ����20mL 0.1mol/L�Ĵ�����Һ�в��ϵ���0.1mol/L��NaOH��Һ����Һ��pH�仯������ͼ��ʾ���ڵζ������У�������Һ������Ũ�ȴ�С��ϵ��������ȷ���ǣ������� ��2013?����һģ������ʱ����20mL 0.1mol?L-1�Ĵ�����Һ�в��ϵ���0.1mol?L-1��NaOH��Һ����Һ��pH�仯������ͼ��ʾ���ڵζ������У�������Һ������Ũ�ȴ�С��ϵ����������ȷ���ǣ�������
��2013?����һģ������ʱ����20mL 0.1mol?L-1�Ĵ�����Һ�в��ϵ���0.1mol?L-1��NaOH��Һ����Һ��pH�仯������ͼ��ʾ���ڵζ������У�������Һ������Ũ�ȴ�С��ϵ����������ȷ���ǣ�������
 ����ʱ����20mL 0.1mol/L�Ĵ�����Һ�в��ϵ���0.1mol/L��NaOH��Һ����Һ��pH�仯������ͼ��ʾ���ڵζ������У�������Һ������Ũ�ȴ�С��ϵ��������ȷ���ǣ� ��
����ʱ����20mL 0.1mol/L�Ĵ�����Һ�в��ϵ���0.1mol/L��NaOH��Һ����Һ��pH�仯������ͼ��ʾ���ڵζ������У�������Һ������Ũ�ȴ�С��ϵ��������ȷ���ǣ� ��