��Ŀ����
��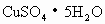 ����500mL 0.2mol/L
����500mL 0.2mol/L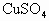 ��Һ�������пո�����д�������������Ƽ�����������
��Һ�������пո�����д�������������Ƽ�����������
(1)��Ҫ����Ҫ�������ƣ�________��
(2)��������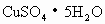 ________g��
________g��
(3)��________ȡ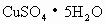 ����________�У���������ˮ����________���裮
����________�У���������ˮ����________���裮
(4)��������Һ��________ע���ݻ�Ϊ________��________�У���������ˮϴ��________��________2��3�Σ���________Ҳ����________�У��ٻ����ؼ���ˮ�����ӽ��̶���________��������________��ˮ��ʹ��Һ��________ǡ����̶���________��
(5)�Ǻ�ƿ������________��סƿ��������һֻ�ֵ���ָ��ס________��������ƿ________��
�𰸣�
������
������
|
(1)������ƽ,ҩ��,�ձ�,������,����ƿ(500mL) ,��ͷ�ι�;(2) 25.0;(3)������ƽ��,�ձ�,������;(4)������,500mL,����ƿ,������,�ձ�,ϴ��Һ,����ƿ,2��3cm,��ͷ�ι�,����,��ƽ;(5)ʳָ,ƿ��,��תҡ������ |

��ϰ��ϵ�д�
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
�����Ŀ