��Ŀ����
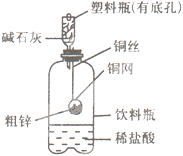
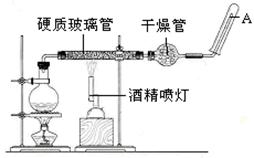
��15�֣� Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��
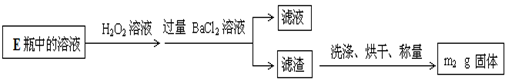
��1��д���÷�Ӧ�Ļ�ѧ����ʽ �������������� �� 8.4g���۲μӷ�Ӧ��ת�Ƶ��� mol��
��2���������ʹ�õĸ�������������������е� ������ţ���
��Ũ���� ��̼���� �ۼ�ʯ�� �ܹ�������
��3��Ӳ�ʲ�������ʯ���������� ��Բ����ƿ��ʢװˮ�� ��������ߵ������� ��
��4��ʵ�鿪ʼʱӦ�ȵ�ȼ ��ʵ�����ʱӦ��Ϩ�� ���������ɵ�����ķ����� ��

��1��д���÷�Ӧ�Ļ�ѧ����ʽ �������������� �� 8.4g���۲μӷ�Ӧ��ת�Ƶ��� mol��
��2���������ʹ�õĸ�������������������е� ������ţ���
��Ũ���� ��̼���� �ۼ�ʯ�� �ܹ�������
��3��Ӳ�ʲ�������ʯ���������� ��Բ����ƿ��ʢװˮ�� ��������ߵ������� ��
��4��ʵ�鿪ʼʱӦ�ȵ�ȼ ��ʵ�����ʱӦ��Ϩ�� ���������ɵ�����ķ����� ��
��15�֣� ��1��3Fe+4H2O(g)  Fe 3O4 +4H2(��д �����ȡ���1�֣�д�ɡ����¡�����д(g)���۷�) ��H2O��0.4 ��2���� ��3������������ˮ�����ĽӴ��� ���Ƭ���ʯ ��ֹ����
Fe 3O4 +4H2(��д �����ȡ���1�֣�д�ɡ����¡�����д(g)���۷�) ��H2O��0.4 ��2���� ��3������������ˮ�����ĽӴ��� ���Ƭ���ʯ ��ֹ����
��4���ƾ��� �ƾ���� ���ռ���������Թܿ��ƽ��ƾ��ƵĻ��棬����������ı����������۵�һ������֤������Ϊ��������˼�Լ��ɣ���
 Fe 3O4 +4H2(��д �����ȡ���1�֣�д�ɡ����¡�����д(g)���۷�) ��H2O��0.4 ��2���� ��3������������ˮ�����ĽӴ��� ���Ƭ���ʯ ��ֹ����
Fe 3O4 +4H2(��д �����ȡ���1�֣�д�ɡ����¡�����д(g)���۷�) ��H2O��0.4 ��2���� ��3������������ˮ�����ĽӴ��� ���Ƭ���ʯ ��ֹ���� ��4���ƾ��� �ƾ���� ���ռ���������Թܿ��ƽ��ƾ��ƵĻ��棬����������ı����������۵�һ������֤������Ϊ��������˼�Լ��ɣ���
��1���ڸ����£�����ˮ������Ӧ����������������������������ʽΪ3Fe+4H2O(g)  Fe 3O4 +4H2��ˮ����Ԫ�صĻ��ϼ۽��ͣ�����ˮ���������� 8.4g����0.15mol������������0.2mol������ת�Ƶ�����0.2mol��2��0.4mol��
Fe 3O4 +4H2��ˮ����Ԫ�صĻ��ϼ۽��ͣ�����ˮ���������� 8.4g����0.15mol������������0.2mol������ת�Ƶ�����0.2mol��2��0.4mol��
��2������ܲ��ܷ�Һ�壬�ٲ���ȷ��̼���Ʋ��Ǹ�������ڲ���ȷ���������ƺ�ˮ��Ӧ�����������������ʣ��ܲ���ȷ��������ȷ�Ĵ�ѡ�ۡ�
��3��ʯ����������������ˮ�����ĽӴ��棬�ӿ췴Ӧ���ʡ�ˮ����ʱ����������������Ҫ�������Ƭ���Է�ֹ���С�
��4��װ���к��п���������Ϊ��ֹ������������������Ҫ����ˮ�����ų������������ȵ�ȼ�ƾ��ơ�ͬ��ʵ�����ʱӦ��Ϩ��ƾ���ơ���������һ���ñ������������ռ���������Թܿ��ƽ��ƾ��ƵĻ��棬����������ı����������۵�һ������֤������Ϊ������
 Fe 3O4 +4H2��ˮ����Ԫ�صĻ��ϼ۽��ͣ�����ˮ���������� 8.4g����0.15mol������������0.2mol������ת�Ƶ�����0.2mol��2��0.4mol��
Fe 3O4 +4H2��ˮ����Ԫ�صĻ��ϼ۽��ͣ�����ˮ���������� 8.4g����0.15mol������������0.2mol������ת�Ƶ�����0.2mol��2��0.4mol����2������ܲ��ܷ�Һ�壬�ٲ���ȷ��̼���Ʋ��Ǹ�������ڲ���ȷ���������ƺ�ˮ��Ӧ�����������������ʣ��ܲ���ȷ��������ȷ�Ĵ�ѡ�ۡ�
��3��ʯ����������������ˮ�����ĽӴ��棬�ӿ췴Ӧ���ʡ�ˮ����ʱ����������������Ҫ�������Ƭ���Է�ֹ���С�
��4��װ���к��п���������Ϊ��ֹ������������������Ҫ����ˮ�����ų������������ȵ�ȼ�ƾ��ơ�ͬ��ʵ�����ʱӦ��Ϩ��ƾ���ơ���������һ���ñ������������ռ���������Թܿ��ƽ��ƾ��ƵĻ��棬����������ı����������۵�һ������֤������Ϊ������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ