��Ŀ����
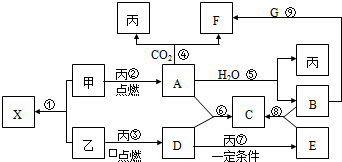
�ס��ҡ���Ϊ�������ʣ��ҡ�����Ԫ�������ڱ���λ��ͬһ���塣X��A��B��C�� D��E��F��G��Ϊ�����Ļ��������A��X ��Ħ��������ͬ��A��F����ɫ��ӦΪ��ɫ����һ�������£��������ת����ϵ��ͼ����ش�

��1��д��ѧʽ����________ E________
��2��X�ĵ���ʽΪ__________��G�Ŀռ乹����__________��
��3��д����Ӧ�ݵĻ�ѧ��Ӧ����ʽ��______________________________��
��4��д��B��Һ��G��Ӧ����F�����ӷ���ʽ��___________________________��
��5������ͼ��-��ķ�Ӧ�У�������������ԭ��Ӧ���ǣ�����ţ�_____________��
��6����8g������������ȼ�գ���������Dͨ��100mL 3.5mol��L-1��B��Һ�У���ȫ���պ���Һ�е����ʼ������ʵ����ֱ�Ϊ______________________����ͬ���������Dͨ��100mL 2.5mol��L-1��B��Һ�У���ȫ���պ���Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ__________________________________��
��2��X�ĵ���ʽΪ__________��G�Ŀռ乹����__________��
��3��д����Ӧ�ݵĻ�ѧ��Ӧ����ʽ��______________________________��
��4��д��B��Һ��G��Ӧ����F�����ӷ���ʽ��___________________________��
��5������ͼ��-��ķ�Ӧ�У�������������ԭ��Ӧ���ǣ�����ţ�_____________��
��6����8g������������ȼ�գ���������Dͨ��100mL 3.5mol��L-1��B��Һ�У���ȫ���պ���Һ�е����ʼ������ʵ����ֱ�Ϊ______________________����ͬ���������Dͨ��100mL 2.5mol��L-1��B��Һ�У���ȫ���պ���Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ__________________________________��
��1��O2��SO3
��2�� ��ֱ����
��ֱ����
��3��2Na2O2+2H2O==4NaOH+O2��
��4��CO2+2OH-==CO32-+H2O
��5�����
��6��0.15molNaHSO3��0.1molNa2SO3��c(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)
��2��
 ��ֱ����
��ֱ���� ��3��2Na2O2+2H2O==4NaOH+O2��
��4��CO2+2OH-==CO32-+H2O
��5�����
��6��0.15molNaHSO3��0.1molNa2SO3��c(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ