��Ŀ����
ʵ����Ҫ��12mol/L��Ũ��������1.0mol/L��ϡ����91mL��
��1��Ҫ����������Һ������
��2�����ƹ����У�����1������ѡ�������Լ��ձ�������������ͷ�ι��⣬�����õ���������
��3��A��B��������Һʱ�����õ��Ķ���������һ���֣�������ṩ���龰��Ҫ��ش����⣮
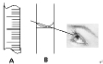
���������������A
������B�����߹۲�Һ�棬����Ҫ�������еIJ�����
�˲������ʱ��������Һ��Ũ��
��1��Ҫ����������Һ������
10mL��Ͳ
10mL��Ͳ
��ȡ12mol/L��Ũ����8.3
8.3
mL����2�����ƹ����У�����1������ѡ�������Լ��ձ�������������ͷ�ι��⣬�����õ���������
100mL����ƿ
100mL����ƿ
����3��A��B��������Һʱ�����õ��Ķ���������һ���֣�������ṩ���龰��Ҫ��ش����⣮

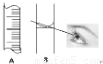
���������������A
��Ͳ
��Ͳ
��B����ƿ
����ƿ
��������B�����߹۲�Һ�棬����Ҫ�������еIJ�����
�ý�ͷ�ιܵμ�����ˮֱ��������ƿ��������
�ý�ͷ�ιܵμ�����ˮֱ��������ƿ��������
���˲������ʱ��������Һ��Ũ��
ƫ��
ƫ��
����ƫ�ߡ�ƫ�͡����������������1��û��91mL����ƿ����ѡ��100mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ������HCl�����ʵ������䣬�ݴ˼�����ҪŨ����������������ҪŨ����������ѡ����Ͳ�Ĺ��
��2������������Һ��ʵ���������ѡ����ȱ�ٵ�������
��3����A��B��������Һʱ�����õ��Ķ���������һ���֣�A���п̶��ߣ���AΪ��Ͳ��Bֻ�п̶��ߣ�BΪ����ƿ��
��ͼ��Ϊ����ʱ����Һ�棬Һ��Ӧ�ڿ̶����Ϸ�������Ҫ������������ˮ��������Һ�����ƫ����ҺŨ��ƫ�ͣ�
��2������������Һ��ʵ���������ѡ����ȱ�ٵ�������
��3����A��B��������Һʱ�����õ��Ķ���������һ���֣�A���п̶��ߣ���AΪ��Ͳ��Bֻ�п̶��ߣ�BΪ����ƿ��
��ͼ��Ϊ����ʱ����Һ�棬Һ��Ӧ�ڿ̶����Ϸ�������Ҫ������������ˮ��������Һ�����ƫ����ҺŨ��ƫ�ͣ�
����⣺��1��û��91mL����ƿ����ѡ��100mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ������HCl�����ʵ������䣬����ҪŨ��������ΪV����V��12mol/L=1.0mol/L��100mL�����V=8.3mL����ѡ����Ϊ10mL��Ͳ���ʴ�Ϊ��10mL��Ͳ��8.3��
��2������12mol/L��Ũ��������1.0mol/L��ϡ����91mL������Ͳ��ȡŨ���ᣬ���ձ���ϡ�ͣ����ò��������裬��ȴ���ò�������������10mL����ƿ�У�ϴ�Ӳ���ϴ��Һ��������ƿ����ˮ���̶���1-2cmʱ�����ý�ͷ�ιܵμ����̶��ߣ��Ǻ�ƿ��ҡ�ȣ���Ҫ������Ϊ����Ͳ���ձ�����������100mL����ƿ����ͷ�ιܵȣ�ȱ�ٵ�����Ϊ��100mL����ƿ��
�ʴ�Ϊ��100mL����ƿ��
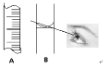
��3����A��B��������Һʱ�����õ��Ķ���������һ���֣�A���п̶��ߣ���AΪ��Ͳ��Bֻ�п̶��ߣ�BΪ����ƿ��
�ʴ�Ϊ����Ͳ������ƿ��
��ͼ��Ϊ����ʱ����Һ�棬Һ��Ӧ�ڿ̶����Ϸ�������Ҫ�ý�ͷ�ιܵμ�����ˮֱ��������ƿ�������У�������Һ�����ƫ����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ���ý�ͷ�ιܵμ�����ˮֱ��������ƿ�������У�ƫ�ͣ�
��2������12mol/L��Ũ��������1.0mol/L��ϡ����91mL������Ͳ��ȡŨ���ᣬ���ձ���ϡ�ͣ����ò��������裬��ȴ���ò�������������10mL����ƿ�У�ϴ�Ӳ���ϴ��Һ��������ƿ����ˮ���̶���1-2cmʱ�����ý�ͷ�ιܵμ����̶��ߣ��Ǻ�ƿ��ҡ�ȣ���Ҫ������Ϊ����Ͳ���ձ�����������100mL����ƿ����ͷ�ιܵȣ�ȱ�ٵ�����Ϊ��100mL����ƿ��
�ʴ�Ϊ��100mL����ƿ��
��3����A��B��������Һʱ�����õ��Ķ���������һ���֣�A���п̶��ߣ���AΪ��Ͳ��Bֻ�п̶��ߣ�BΪ����ƿ��
�ʴ�Ϊ����Ͳ������ƿ��
��ͼ��Ϊ����ʱ����Һ�棬Һ��Ӧ�ڿ̶����Ϸ�������Ҫ�ý�ͷ�ιܵμ�����ˮֱ��������ƿ�������У�������Һ�����ƫ����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ���ý�ͷ�ιܵμ�����ˮֱ��������ƿ�������У�ƫ�ͣ�
���������⿼��һ�����ʵ���Ũ����Һ�����ƣ��Ѷ��еȣ�ע��������Һ���Ƶ�ԭ������3���Т�Ϊ�״��㣬ע��������Ŀ��Ҫ��ϸ�Ĺ۲죮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��ͼ��ʾװ�ÿ����ڶ���ʵ�飮
��ͼ��ʾװ�ÿ����ڶ���ʵ�飮