��Ŀ����
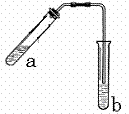
����ͼʾװ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ������գ�
��1���Թ�a����Ҫ����Ũ���ᡢ��������Ҵ���2mL����
ȷ����˳���������������������������������������
_____________________________________________��Ϊ��ֹa�е�Һ����ʵ��ʱ�������У��ڼ���ǰӦ��ȡ�Ĵ�ʩ�� ____________
��2��д���Թ�a�и÷�Ӧ��ѧ����ʽ������������������������������������ ��
��3��ʵ���м����Թܣ�
�� ��ʼʱҪС����ȵ�Ŀ���ǣ�___________________________________________________________________
�ڼ���һ��ʱ����Ϊ�����������ڵ�Ŀ���ǣ�__________________________________________________________________
���� ![]() ���������ں��ٸ�ΪС����ȵ�Ŀ���ǣ�
���������ں��ٸ�ΪС����ȵ�Ŀ���ǣ�
�� ������������������������������������������������������������������
��4���Թ�b�м��б���Na2CO3��Һ ���������ǣ�
_____________________________������������������ ��������������������
��5���Թ�b�е����ܲ�����Һ���µ�Ŀ���ǣ� ___________________________
��1���ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ���ᣬ��ȴ���ټӱ����ᡡ����
���Թ�a�м��뼸����ʯ�������Ƭ��(��1��)��2���ԣ�(2��)����������
��3�� �ټ����Ҵ�������Ļӷ� ���� �ڼ�ʱ��������������������������ƽ�����������������ķ����ƶ� ![]() ���ٸ���Ӧ����(��1��)
���ٸ���Ӧ����(��1��)
��4���������������������������������ʺ��Ҵ�,���������������ܽ��(2��)
��5����ֹ���� (1��)������������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ����ͼʾװ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ������գ�
����ͼʾװ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ������գ� 23������ͼʾװ����ȡ�������������������
23������ͼʾװ����ȡ�������������������
