��Ŀ����
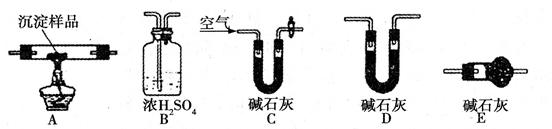
(14��)(��)����4�֣�ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ�������ʣ�O2��H2CrO4��Cr(OH)3��H2O��H2O2
��֪�÷�Ӧ��H2O2ֻ�������¹��̣�H2O2�� O2
��1��д���÷�Ӧ�Ļ�ѧ����ʽ________________________��
��2���÷�Ӧ�еĻ�ԭ���� ����ԭ������____________��
(��)��10�֣���ֻ�Լ�ƿ�зֱ�ʢװ��NaNO3��Һ��Na2CO3��Һ��Na2SO4��Һ��NaCl��Һ������μ�����������Һ�ֱ������и��⡣
����֧�Թ��зֱ�ȡ������Һ��1mL��������ʵ�顣
��1������֧�Թ��зֱ���� ������ ����ģ��� ��
��2����ʣ����֧�Թ��зֱ���� ������ ����ģ��� ��
��3����ʣ����֧�Թ��зֱ���� ������ ����ģ��� ������ʵ���ж�û������������� ��
����:

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�(14��)ijͬѧ����ˮ�ʼ��վ����960mL 1 mol��L��1NaOH��Һ�Ա�ʹ�á�
(1)��ͬѧӦѡ��________mL������ƿ��
(2)�������������ͼ��ʾ������ͼ����Ӧ����ͼ�е�________(��ѡ����ĸ)֮�䡣
A������ۡ�������B������ڡ�������C�������
(3)��ͬѧӦ��ȡNaOH����________g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С________(����ĸ)��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��________(����ĸ)��
������������
| | a | b | c | d | e |
| �����С/g | 100 | 50 | 20 | 10 | 5 |

(4)���в�����������Һ��Ũ�ȴ�С�к�Ӱ�죿
��ת������Һ��δϴ�Ӳ��������ձ���Ũ�Ȼ�__________(�ƫ��ƫС������Ӱ�족����ͬ)
������ƿ��ԭ������������ˮ��Ũ�Ȼ�____________��
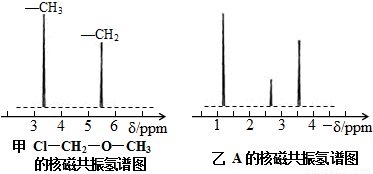
 λ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ���źţ������ݷ�ֵ���źţ�����ȷ����������ԭ�ӵ��������Ŀ�����磺���ȼ��ѣ�Cl�DCH2�DO�DCH3����������ԭ�ӣ�����ͼ�������ⶨ���л���A�ĺ˴Ź�������ͼ������ͼ����A�Ľṹ��ʽΪ ��
λ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ���źţ������ݷ�ֵ���źţ�����ȷ����������ԭ�ӵ��������Ŀ�����磺���ȼ��ѣ�Cl�DCH2�DO�DCH3����������ԭ�ӣ�����ͼ�������ⶨ���л���A�ĺ˴Ź�������ͼ������ͼ����A�Ľṹ��ʽΪ ��