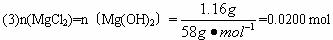��Ŀ����
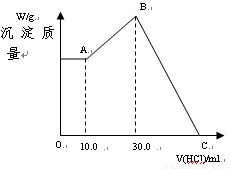
��16�֣���NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ������������������Һ����μ���1.00mol/L HCl��Һ������HCl��Һ����������ɳ����Ĺ�ϵ��ͼ��ʾ��
�Իش�
��1��A��ij�����Ļ�ѧʽΪ ������ ��
��2��д��A�㵽B�㷢����Ӧ�����ӷ���ʽ ��
��3��ԭ�������MgCl2�������� ��AlCl3�������� ��NaOH�������� ��
��4��C����Һ�����Ϊ mL��
��16�֣�
(1)Mg(OH)2 ��2�֣������������ǡ���к��������ơ���2�֣�
(2) AlO2-+ H+ + H2O = Al(OH)3����2�֣�(3) 1.90 g ��2�֣���2.67 g��2�֣���5.20 g��3�֣� ��4��130 ml��3�֣�
����:��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
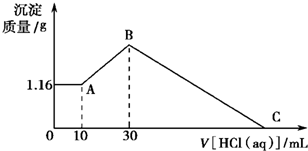 ��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ�����������õ�����Һ����μ���1mol?L-1 HCl��Һ������HCl��Һ�����V�����ɳ���������m�Ĺ�ϵ��ͼ��ʾ���Իش�?
��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ�����������õ�����Һ����μ���1mol?L-1 HCl��Һ������HCl��Һ�����V�����ɳ���������m�Ĺ�ϵ��ͼ��ʾ���Իش�?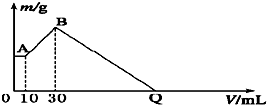 ��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ����������������Һ����μ���1mol?L-1 HCl��Һ������HCl��Һ����������ɳ�����������ϵ��ͼ��ʾ���Իش�
��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ����������������Һ����μ���1mol?L-1 HCl��Һ������HCl��Һ����������ɳ�����������ϵ��ͼ��ʾ���Իش�