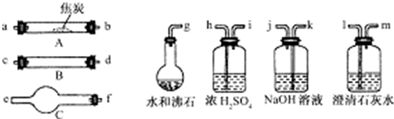
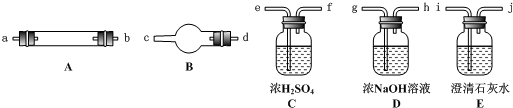
��Ŀ����
28.ˮ����ͨ�����ȵĽ�̿�������������Ҫ�ɷ���CO��H2������CO2��ˮ�����ȡ�������ͼ���ṩ��������ѡ���Ҫ���Լ������һ��ʵ�飬֤���������������CO��H2��������װ�ú͵��ܵ���ͼ����ȥ��
�ش��������⣺
��1��ʢŨH2SO4��װ����;�� ��ʢNaOH��Һ��װ����;�� ��
��2������B��������Լ������ƣ���ѧʽ���ǣ� ����������Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��3������C��������Լ������ƣ���ѧʽ���ǣ� ����Ŀ���� ��
��4���������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��g��ab�� ��
��5����֤��������к���CO��ʵ�������� ��
��6����֤��������к���H2��ʵ�������� ��
(1)��ȥˮ���� ��ȥCO2
(2)����ͭ(CuO)
CuO+H2=Cu+H2O CuO+CO=Cu+CO2
(3)��ˮ����ͭ(CuSO4) ����H2O
(4)(g��ab)��kj��hi��cd(��dc)��fe��lm
(5)ԭ������е�CO2�ѱ���ȥ������CO��CuO��Ӧ���ɵ�CO2ʹ����ʯ��ˮ�����
(6)ԭ������е�H2O�ѱ���ȥ������H2��CuO��Ӧ���ɵ�H2Oʹ��ˮ����ͭ�ɰ�ɫ��Ϊ��ɫ
����������Ϊһ�ۺϿ������ʵ��Ʊ������ӡ����飬���������ӵ����ݵ��ۺ�ʵ���⡣����ˮ������ˮ����ͨ��A�н�̿����CO��H2��CO2��H2O(g)�����壬���Ⱦ���NaOH��Һ��ȥCO2(�������CO�ļ���)����ͨ��ŨH2SO4��ȥˮ�������ų��˶�H2����ĸ��ţ���ͨ��ʢ������CuO��B�ܷ�����ӦH2+CuO![]() Cu+H2O��CO+CuO
Cu+H2O��CO+CuO![]() Cu+CO2���ٽ�����������ͨ��ʢ��CuSO4�ĸ����C�����ɰ�ɫ��ĩ����ȷ����H2���������ͨ�����ʯ��ˮ������ǣ���֤����CO����ϴ��װ�ó����̳��������װ�ô��С������ȷ����ӿ�˳��
Cu+CO2���ٽ�����������ͨ��ʢ��CuSO4�ĸ����C�����ɰ�ɫ��ĩ����ȷ����H2���������ͨ�����ʯ��ˮ������ǣ���֤����CO����ϴ��װ�ó����̳��������װ�ô��С������ȷ����ӿ�˳��