��Ŀ����
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϣ�
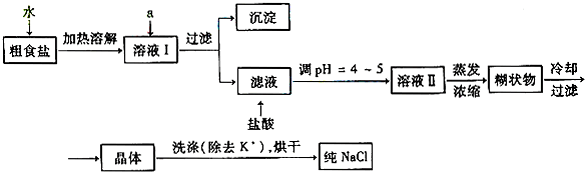
��1����ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�

�ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��ҺBa��NO3��2��Һ��75%�Ҵ������Ȼ�̼
������ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ______��ֻ�ѧʽ����
��ϴ�ӳ�ȥNaCl������渽��������KCl��ѡ�õ��Լ�Ϊ______��
��2�����ᴿ��NaCl����500mL4.00mol?L-1NaCl��Һ������������ҩ�ס����������______�����������ƣ���
��3����ⱥ��ʳ��ˮ��װ����ͼ��ʾ�����ռ���H2Ϊ2L����ͬ���������ռ���Cl2______�����������=��������2L��ԭ����______��װ�øĽ��������Ʊ�NaOH��Һ�����ⶨ��Һ��NaOH��Ũ�ȣ����õķ�����______��
��4��ʵ�����Ʊ�H2��Cl2ͨ���������з�Ӧ��
Zn+H2SO4����ZnSO4+H2����MnO2+4HCl��Ũ�� MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
�ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��______������ţ����Ʊ����ռ��������Cl2��װ��______������ţ�����ѡ���Ʊ������װ�ã�

�⣺��1����Ҫ��ȥSO42-��ֻ��ѡBaCl2��Һ����ѡ��Ba��NO3��2���������µ�����NO3-����ѡ��NaOH��Һ��ȥMg2+��Fe3+��Һ�����ѡ��Na2CO3��Һ��ȥCa2+���˴�����ѡ��K2CO3��Һ������������µ�K+������HCl��ȥ������CO32-��Na2CO3��Һ���ܼ���BaCl2��Һǰ�����������Ba2+���ʴ�Ϊ��BaCl2��NaOH��Na2CO3��
�ڳ�ȥNaCl��������������KCl��Ӧѡ��75%���Ҵ�����ΪCCl4�ж���ͬʱKClҲ�����ܽ���CCl4�У�
�ʴ�Ϊ��75%�Ҵ���
��2������һ�����ʵ���Ũ�ȵ���Һ������������У�ҩ�ס�����������ƽ���ձ���500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����ƽ���ձ���500mL����ƿ����ͷ�ιܣ�
��3����ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H2O 2NaOH+Cl2��+H2�������������ڲ�����Cl2����������ɵ�NaOH��ӦNaCl��NaClO��H2O��ʹ�ò��ֵ�Cl2�����ģ�����ͬ���������ռ�����Cl2С��2L�����ⶨ��Һ��NaOH��Ũ�ȣ������к͵ζ��ķ�����
2NaOH+Cl2��+H2�������������ڲ�����Cl2����������ɵ�NaOH��ӦNaCl��NaClO��H2O��ʹ�ò��ֵ�Cl2�����ģ�����ͬ���������ռ�����Cl2С��2L�����ⶨ��Һ��NaOH��Ũ�ȣ������к͵ζ��ķ�����
�ʴ�Ϊ������������ɵ������������ɵ�NaOH�����˷�Ӧ���к͵ζ���
��4��ʵ�����в��ý���п������ȷ�Ӧ�������������ѡ��װ��e�����ö������̺�Ũ���������������������е������Ȼ�����Լӱ���ʳ��ˮ����������ˮ���Բ���Ũ��������ȥ������ѡ��װ��d���ʴ�Ϊ��e��d��
��������1��������ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO42-���ӣ����Էֱ����̼���ơ��������ơ��������ƺ��Ȼ����������Լ�������ԭ�����ش�
�ڸ����л���������Լ��Ȼ������л����е��ܽ��֪ʶ���ش�
��2������һ�����ʵ���Ũ�ȵ���Һ������������У�ҩ�ס�����������ƽ���ձ���500mL����ƿ����ͷ�ιܣ�
��3�����ݵ�ⱥ��ʳ��ˮʱ�IJ����Լ��������������Ƶķ�Ӧ�Լ��к͵ζ�ʵ��ԭ��֪ʶ���ش�
��4��ʵ�����в��ý���п���ᷴӦ��������������ö������̺�Ũ������������������
������������Ҫ�������ڴ����ᴿ�Ĺ�������ѡ�ó��Ӻ;����ķ�������ȥ���ʲ�Ҫ�����µ����ʣ�����ʵ������й������Լ���Ҫ��ȥ��
�ڳ�ȥNaCl��������������KCl��Ӧѡ��75%���Ҵ�����ΪCCl4�ж���ͬʱKClҲ�����ܽ���CCl4�У�
�ʴ�Ϊ��75%�Ҵ���
��2������һ�����ʵ���Ũ�ȵ���Һ������������У�ҩ�ס�����������ƽ���ձ���500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����ƽ���ձ���500mL����ƿ����ͷ�ιܣ�
��3����ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H2O
 2NaOH+Cl2��+H2�������������ڲ�����Cl2����������ɵ�NaOH��ӦNaCl��NaClO��H2O��ʹ�ò��ֵ�Cl2�����ģ�����ͬ���������ռ�����Cl2С��2L�����ⶨ��Һ��NaOH��Ũ�ȣ������к͵ζ��ķ�����
2NaOH+Cl2��+H2�������������ڲ�����Cl2����������ɵ�NaOH��ӦNaCl��NaClO��H2O��ʹ�ò��ֵ�Cl2�����ģ�����ͬ���������ռ�����Cl2С��2L�����ⶨ��Һ��NaOH��Ũ�ȣ������к͵ζ��ķ������ʴ�Ϊ������������ɵ������������ɵ�NaOH�����˷�Ӧ���к͵ζ���
��4��ʵ�����в��ý���п������ȷ�Ӧ�������������ѡ��װ��e�����ö������̺�Ũ���������������������е������Ȼ�����Լӱ���ʳ��ˮ����������ˮ���Բ���Ũ��������ȥ������ѡ��װ��d���ʴ�Ϊ��e��d��
��������1��������ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO42-���ӣ����Էֱ����̼���ơ��������ơ��������ƺ��Ȼ����������Լ�������ԭ�����ش�
�ڸ����л���������Լ��Ȼ������л����е��ܽ��֪ʶ���ش�
��2������һ�����ʵ���Ũ�ȵ���Һ������������У�ҩ�ס�����������ƽ���ձ���500mL����ƿ����ͷ�ιܣ�
��3�����ݵ�ⱥ��ʳ��ˮʱ�IJ����Լ��������������Ƶķ�Ӧ�Լ��к͵ζ�ʵ��ԭ��֪ʶ���ش�
��4��ʵ�����в��ý���п���ᷴӦ��������������ö������̺�Ũ������������������
������������Ҫ�������ڴ����ᴿ�Ĺ�������ѡ�ó��Ӻ;����ķ�������ȥ���ʲ�Ҫ�����µ����ʣ�����ʵ������й������Լ���Ҫ��ȥ��

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ