��Ŀ����
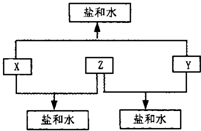
��֪����A��B��C��D�������ʾ���Ԫ��X���еĻ����ܺ���Ԫ��Y��Z��Ԫ��Y��X��Z��ԭ���������ε�������X��A��B��C��D�ж���������������ϼۣ��������µ���A��ij�ֳ���һԪǿ����Һ��Ӧ���ɵõ�B��C���ܻ�����D���ȴ��ֽ⣬���Ƶ�Ԫ��Y�ĵ��ʣ�
(1)Ԫ��X��________��Z��________
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
________________________________________________________
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
________________________________________________________
�𰸣�
������
������
|
(1)Cl(����),K(���) (2)Cl2��2KOH (3)2KClO3 |

��ϰ��ϵ�д�
�����Ŀ
H2��I2��һ���������ܷ�����Ӧ��H2��g��+I2��g��?2HI��g����H=-a kJ?mol-1
��֪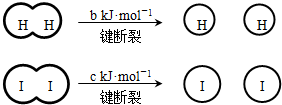 ��a��b��c�������㣩
��a��b��c�������㣩
����˵������ȷ���ǣ�������
��֪
 ��a��b��c�������㣩
��a��b��c�������㣩����˵������ȷ���ǣ�������
| A����Ӧ�������������������������� | B���Ͽ�1mol H-H����1mol I-I�������������ڶϿ�2mol H-I���������� | C���Ͽ�2mol H-I����������ԼΪ��c+b+a��kJ | D�����ܱ������м���2mol H2��2mol I2����ַ�Ӧ��ų�������С��2a kJ |

 ��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
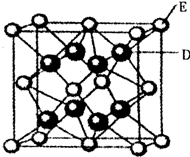 ��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�
��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�