��Ŀ����
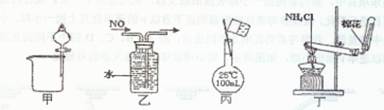
ij�ֺ�������FeCl2���ʵ�FeCl3��Ʒ����Ҫ�ⶨ������Ԫ�صĺ�����ʵ��������²�����У�
1��ȷ����m g��Ʒ��2��3g����
2������Ʒ�м���10mL 5mol/L�����ᣬ�ټ�������ˮ�����Ƴ�250mL��Һ��
3����ȡ25mL����������õ���Һ������3mL��ˮ������ʹ֮��ȫ��Ӧ��
4������Ѹ�ټ���Ũ��Ϊ10%�İ�ˮ����������ֽ��裬ʹ֮��ȫ������
5�����ˣ�������ϴ�ӡ����ա���ȴ�������������������أ�
���������������ش�
��1��������ǰ��������ƽ��ָ��ƫ��������������ʱ��ָ��պ��ڱ�ߵ��м䣬��������Ʒ������______��
A����mg�ࡡ���� B����mg�١�������C��ǡ��Ϊmg
��2���ܽ���ƷʱҪ�������ᣬԭ����______��
��3������250mL��Һʱ������250mL������ƿ���ձ��⣬�����õ��IJ���������______��
��4��������ˮʱ������Ӧ�����ӷ���ʽ��______��
��5������������ΪW1 g�����������պ�������������W2g������Ʒ����Ԫ�ص�����������______��
��6����������250mL��Һʱ�����õ�����ƿû��ϴ�ɾ�����������������ʱ�����ջ�ʹ��Ԫ�صIJⶨ�������ƫ�ߡ�����ƫ�͡����䡱������NaCl______��AlCl3______��
�⣺��1������ǰ����ƽָ��ƫ��˵��������������������������������ʱ��ָ��պ��ڱ�ߵ��м䣬��������Ʒ������ƫС����ѡ��B��
��2��Fe2+��Fe3+�ܷ���ˮ�⣬�ʴ�Ϊ������Fe2+��Fe3+��ˮ�⣻
��3�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ������������ͷ�ιܣ�
��4����������������ԣ�������Fe2+����Fe3+��2Fe2++Br2=2Fe3++2Br-���ʴ�Ϊ��2Fe2++Br2=2Fe3++2Br-��
��5�������������ȷֽ⣺2Fe��OH��3 Fe2O3+3H2O����֪���պ����ΪFe2O3��Fe2O3������Ϊ��W2-W1������Ԫ�ص�����Ϊ��
Fe2O3+3H2O����֪���պ����ΪFe2O3��Fe2O3������Ϊ��W2-W1������Ԫ�ص�����Ϊ��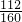 ��W2-W1������Ʒ������������
��W2-W1������Ʒ������������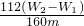 ��100%=
��100%=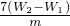 %��
%��
�ʴ�Ϊ��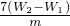 %��
%��
��6���ٵ�����NaClʱ�����������IJ��仯�����Բ�Ӱ�������ʴ�Ϊ�����䣻
�ڵ�����NaClʱ�����������ı��������Ԫ�صIJⶨ����ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��������1������ʹ��������ƽ����ҩƷʱӦ��ѭ���������롱����ƽָ��ƫ��˵����Ʒ������������������������
��2������Fe2+��Fe3+�ܷ���ˮ�⣻
��3������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ�������������
��4�����ݵ�������������ԣ�������Fe2+����Fe3+��
��5�����������������ȷֽ⣺2Fe��OH��3 Fe2O3+3H2O����֪���պ����ΪFe2O3��������Ԫ���غ㣬��֪��Ԫ�ص������������Ʒ����Ԫ�ص�����������
Fe2O3+3H2O����֪���պ����ΪFe2O3��������Ԫ���غ㣬��֪��Ԫ�ص������������Ʒ����Ԫ�ص�����������
��6�����������Ƿ�������������ı仯��������
������������Ҫ��������Ԫ�ص����������IJⶨ��ͬʱ������ʵ��֪ʶ���ѶȲ���
��2��Fe2+��Fe3+�ܷ���ˮ�⣬�ʴ�Ϊ������Fe2+��Fe3+��ˮ�⣻
��3�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ������������ͷ�ιܣ�
��4����������������ԣ�������Fe2+����Fe3+��2Fe2++Br2=2Fe3++2Br-���ʴ�Ϊ��2Fe2++Br2=2Fe3++2Br-��
��5�������������ȷֽ⣺2Fe��OH��3
 Fe2O3+3H2O����֪���պ����ΪFe2O3��Fe2O3������Ϊ��W2-W1������Ԫ�ص�����Ϊ��
Fe2O3+3H2O����֪���պ����ΪFe2O3��Fe2O3������Ϊ��W2-W1������Ԫ�ص�����Ϊ��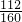 ��W2-W1������Ʒ������������
��W2-W1������Ʒ������������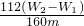 ��100%=
��100%=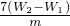 %��
%���ʴ�Ϊ��
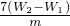 %��
%�� ��6���ٵ�����NaClʱ�����������IJ��仯�����Բ�Ӱ�������ʴ�Ϊ�����䣻
�ڵ�����NaClʱ�����������ı��������Ԫ�صIJⶨ����ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��������1������ʹ��������ƽ����ҩƷʱӦ��ѭ���������롱����ƽָ��ƫ��˵����Ʒ������������������������
��2������Fe2+��Fe3+�ܷ���ˮ�⣻
��3������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ�������������
��4�����ݵ�������������ԣ�������Fe2+����Fe3+��
��5�����������������ȷֽ⣺2Fe��OH��3
 Fe2O3+3H2O����֪���պ����ΪFe2O3��������Ԫ���غ㣬��֪��Ԫ�ص������������Ʒ����Ԫ�ص�����������
Fe2O3+3H2O����֪���պ����ΪFe2O3��������Ԫ���غ㣬��֪��Ԫ�ص������������Ʒ����Ԫ�ص�������������6�����������Ƿ�������������ı仯��������
������������Ҫ��������Ԫ�ص����������IJⶨ��ͬʱ������ʵ��֪ʶ���ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
�±����и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת������
| ���� ��� | ����ת����ϵ | �� | �� | �� | �� |
| �� | 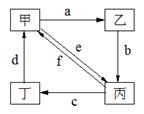 | Cu | CuO | CuSO4 | Cu(NO3)2 |
| �� | Na2CO3 | NaOH | NaHCO3 | CO2 | |
| �� | (NH4)2SO3 | CaSO3 | SO2 | NH4HSO3 | |
| �� | CH3CH2Cl | C2H5OH | CH2=CH2 | CH3CH3 |
- A.�٢ڢۢ�
- B.�٢ڢ�
- C.�٢ۢ�
- D.�ڢ�
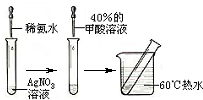 ���ᣨCH2O2�����׳����ᣬ�ṹʽΪ��
���ᣨCH2O2�����׳����ᣬ�ṹʽΪ��