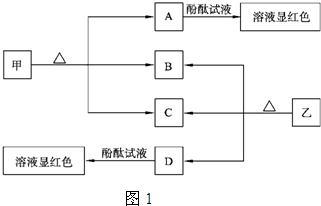
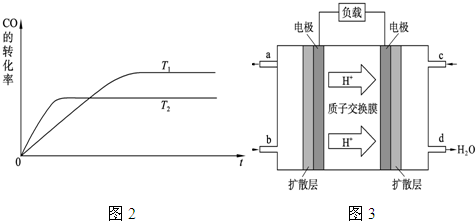
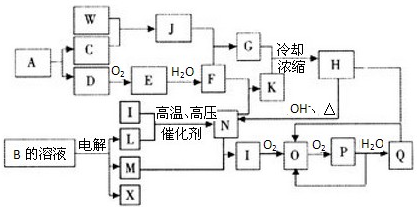
��Ŀ����
���³�ѹ�£������и�������ֱ���뵽�ݻ��ɱ���ܱ������У���ַ�Ӧ�ָ���ԭ��״��ʱ����ɴ�С��˳���ǣ���2mol NH3��1mol HCl����2mol SO2��1mol O2����2molH2S��1mol SO2����2mol NO��1mol O2����1mol H2S��1mol Cl2��������
�������ȿ������߷�����Ӧ��Ȼ�������ͬ״���£�����������С����������ʵ���������
����⣺����NH3��HCl������Ӧ��NH3+HCl=NH4Cl��NH3������ʣ����������ʵ���Ϊ1mol��
����ȻSO2��O2�ڳ��³�ѹ�º��ѷ������ܱ�����������ΪSO2��O2��������ܵ����ʵ���Ϊ3mol��
����H2S�� SO2������Ӧ��2H2S+SO2=3S+2H2O������ǡ�÷�Ӧ��SΪ���壬H2OΪҺ�壬�ܱ�������û�����壻
����NO��O2������Ӧ��2NO+O2=2NO2������ǡ�÷�Ӧ����NO2����ת���N2O4��2NO2?N2O4���ܱ�������������NO2��N2O4�����ʵ�������1molС��2mol��
����H2S��Cl2������Ӧ��H2S+Cl2=2HCl+S������ǡ�÷�Ӧ���ܱ�����������ֻ��HCl�����ʵ���Ϊ2mol��
��ˣ�����ɴ�С��˳�ڣ��ݣ��ܣ��٣��ۣ�
��ѡB��
����ȻSO2��O2�ڳ��³�ѹ�º��ѷ������ܱ�����������ΪSO2��O2��������ܵ����ʵ���Ϊ3mol��
����H2S�� SO2������Ӧ��2H2S+SO2=3S+2H2O������ǡ�÷�Ӧ��SΪ���壬H2OΪҺ�壬�ܱ�������û�����壻
����NO��O2������Ӧ��2NO+O2=2NO2������ǡ�÷�Ӧ����NO2����ת���N2O4��2NO2?N2O4���ܱ�������������NO2��N2O4�����ʵ�������1molС��2mol��
����H2S��Cl2������Ӧ��H2S+Cl2=2HCl+S������ǡ�÷�Ӧ���ܱ�����������ֻ��HCl�����ʵ���Ϊ2mol��
��ˣ�����ɴ�С��˳�ڣ��ݣ��ܣ��٣��ۣ�
��ѡB��
������������Ҫ��������ͬ״���£���������ʵ���Խ�����Խ��ͬʱ��Ҫ���Ƿ�Ӧ�Ƿ����Լ�������Ӧ�ķ�����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ