��Ŀ����
����Ŀ��̫���ܵ����ͨ�����ЧӦ���߹⻯ѧЧӦֱ�Ӱѹ���ת���ɵ��ܵ�װ�á�����ϳ������裬����ͭ�������ء����Ȼ����
��1���صĻ�̬ԭ�ӵļ۵����Ų�ʽ��___________��
��2����֪��Ϊ��4���ڢ�A��Ԫ�أ������������ڵ�ͬ����Ԫ���У���һ�����ܴӴ�С˳��Ϊ___����Ԫ�ط��ű�ʾ����
��3����̬SeO3���ӵ����幹��Ϊ_____________________��
��4������(SinH2n+2)�ķе�������Է��������ı仯��ϵ��ͼ��ʾ���������ֱ仯��ϵ��ԭ���ǣ�_________��

��5������Ԫ�ش���ͬһ�������Ԫ�ؾ���ȱ�����ԣ��仯�����������мӺ��ԣ��������(H3BO3)��ˮ��Һ������ˮ��Ӧ����[B(OH)4]��������һԪ��������ʣ���[B(OH)4]����B��ԭ���ӻ�����Ϊ______��
��6������Cu�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ����Ӧ�����ӷ���ʽΪ_______��
��7��һ��ͭ��Ͻ�����������������ܶѻ��Ľṹ.�ھ����У�Auԭ��λ�ڶ��㣬Cuԭ��λ�����ģ���úϽ���Auԭ����Cuԭ�Ӹ���֮��Ϊ______����Au��ԭ�Ӱ뾶Ϊa pm��Cu��ԭ�Ӱ뾶Ϊb pm����Ͻ���ܶ�Ϊ________g��cm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
���𰸡�4s24p1 Br��As��Se ƽ�������� �������Է�������Խ���Ӽ䷶�»���Խǿ sp3 Cu+H2O2+4NH3��H2O=Cu(NH3)42++2OH��+4H2O 1:3 ![]()
��������
(1)����31��Ԫ�أ�����ԭ�Ӻ�������Ų����ɿ���д���۵����Ų���
(2)�顢����������Ԫ�ض��ǵ�4���ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ�����Ԫ��ԭ��4p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������Br��As��Se���ݴ˴��⣻
(3)��̬SeO3����������ԭ�ӵļ۲���Ӷ��������жϷ��ӹ��ͣ�
(4)���飨SinH2n+2�����Ƿ��Ӿ��壬���Ӿ���ķе�ߵ�ȡ���ڷ��Ӽ��������������Ӽ�����������Է��������Ĵ�С�йأ��ݴ˴��⣻
(5)���ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
(6)����������ԭ��Ӧ��Ԫ�غ͵���غ㣬��д�����ӷ���ʽ��
(7)���þ�̯���������ֽ���ԭ�Ӹ���֮�ȣ�����![]() ���㡣
���㡣
(1)����31��Ԫ�أ�����ԭ�Ӻ�������Ų����ɿ���д���۵����Ų�Ϊ��4s24p1 ��
��ˣ�������ȷ���ǣ�4s24p1��
(2)�顢����������Ԫ�ض��ǵ�4���ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ�����Ԫ��ԭ��4p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������Br��As��Se��
��ˣ�������ȷ���ǣ�Br��As��Se��
(3)��̬SeO3����������ԭ�ӵļ۲���Ӷ���Ϊ![]() =3���µ��Ӷԣ����Է��ӹ���Ϊƽ�������Σ�
=3���µ��Ӷԣ����Է��ӹ���Ϊƽ�������Σ�
��ˣ�������ȷ���ǣ�ƽ�������Σ�
(4)���飨SinH2n+2�����Ƿ��Ӿ��壬���Ӿ���ķе�ߵ�ȡ���ڷ��Ӽ��������������Ӽ�����������Է��������Ĵ�С�йأ��������Է�������Խ���Ӽ䷶�»���Խǿ��
��ˣ�������ȷ���ǣ��������Է�������Խ���Ӽ䷶�»���Խǿ��
(5) [B(OH)4]����B�ļ۲���Ӷ�=4+![]() ��3+1-4
��3+1-4![]() 1��=4�����Բ�ȡsp3�ӻ���
1��=4�����Բ�ȡsp3�ӻ���
��ˣ�������ȷ���ǣ�sp3��
(6)����Cu�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ��˵�������ܻ���ٽ������������ʹ�ͬ���õĽ�������й�������Ϊ������������Cu2+�γ������ӣ�������ٽ�ʹ��Ӧ���У�����ʽ�ɱ�ʾΪ��Cu+H2O2+4NH3��H2O=Cu(NH3)42++2OH��+4H2O��
��ˣ�������ȷ���ǣ�Cu+H2O2+4NH3��H2O=Cu(NH3)42++2OH��+4H2O ��
(7)�ھ����У�Auԭ��λ�ڶ��㣬Cuԭ��λ�����ģ��þ�����Auԭ�Ӹ���=8![]() =1��Cuԭ�Ӹ���=6
=1��Cuԭ�Ӹ���=6![]() =3�����ԸúϽ���Auԭ����Cuԭ�Ӹ���֮��=1��3��
=3�����ԸúϽ���Auԭ����Cuԭ�Ӹ���֮��=1��3��
��Au��ԭ�Ӱ뾶Ϊa pm��Cu��ԭ�Ӱ뾶Ϊb pm���������ⳤ=![]() cm��
cm��
�������V=(![]() cm)3��ÿ��������ͭԭ�Ӹ�����3��Auԭ�Ӹ�����1����
cm)3��ÿ��������ͭԭ�Ӹ�����3��Auԭ�Ӹ�����1����![]() =
=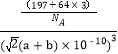 g��cm-3=
g��cm-3=![]() g��cm-3��
g��cm-3��
��ˣ�������ȷ���ǣ�1��3��![]() ��
��




