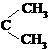��Ŀ����
27.ϩ��ͨ������������п��ˮ�����õ�ȩ��ͪ�����磺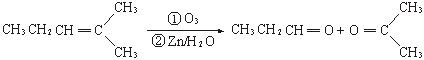
��.��֪��ȩ��ȼ����Ϊ1815 kJ/mol����ͪ��ȼ����Ϊ1789 kJ/mol����д����ȩȼ�յ��Ȼ�ѧ����ʽ ��
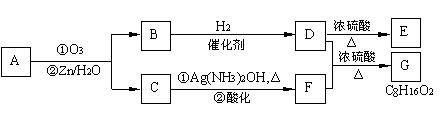
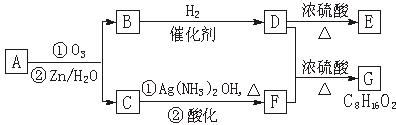
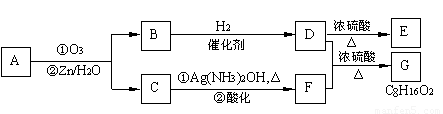
��.������Ӧ�������ƶ�ϩ���Ľṹ��һ����״��ϩ��Aͨ������������п��ˮ�����õ�B��C��������B��̼69.8%������11.6%��B��������Ӧ������������D��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E����Ӧͼʾ���£�
�ش��������⣺
��1��B����Է��������� ��C��F�ķ�Ӧ����Ϊ ��D�к��й����ŵ����� ��
��2��D+F��G�Ļ�ѧ����ʽ�� ��
��3��A�Ľṹ��ʽΪ ��
��4��������A��ij��ͬ���칹��ͨ������������п��ˮ����ֻ�õ�һ�ֲ�����ϸ��������칹��Ľṹ��ʽ�� �֡�
�𰸣�
��.CH3CH2CHO��l��+4O2��g��===3CO2��g��+3H2O��l������H=��1815 kJ/mol
��.��1��86 ������Ӧ �ǻ�
��2��CH3CH2COOH����C2H5��2CHOH ![]() CH3CH2COOCH��C2H5��2��H2O
CH3CH2COOCH��C2H5��2��H2O
��3����CH3CH2��2C=CHCH2CH3
��4��3

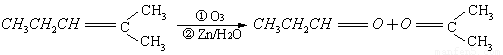
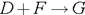 ��
��

 ����ͪ��ȼ����Ϊ1789
����ͪ��ȼ����Ϊ1789 ����д����ȩȼ�յ��Ȼ�ѧ����ʽ_____________ ��
����д����ȩȼ�յ��Ȼ�ѧ����ʽ_____________ ��