��Ŀ����
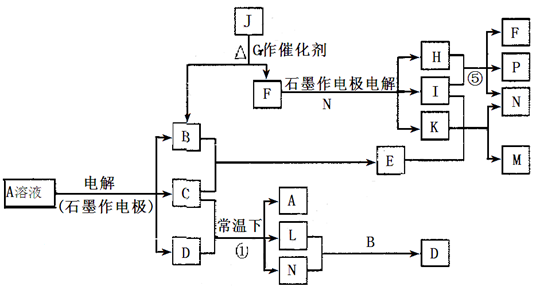
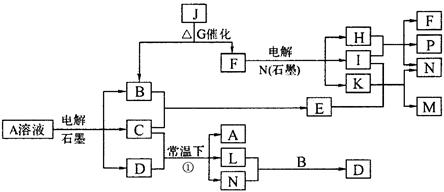
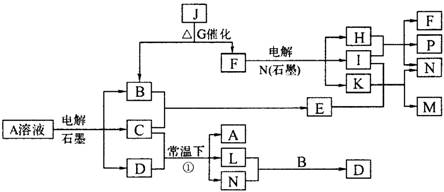
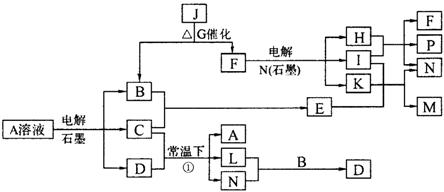
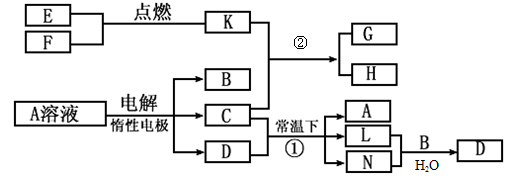
��֪AΪ��ɫ��Һ��B��C��E��FΪ���ʣ������Ϊ���������B��L��F������Ϊ���壬��FΪ��ɫ���壬C��EΪ������K��ˮ��ҺΪ��ɫ��������ת����ϵ��ͼ��
�ش��������⣺
��1����Ӧ�ڵĻ�ѧ����ʽ��____________ ��
��2����Ӧ�ٵ����ӷ���ʽ��_________________��
��3���ö��Ե缫���400.00mL A��Һ��һ��ʱ���ڲ����ҺpH��1������Ҫ����Һ�м���__________��������Ϊ______g������ʹ��Һ�ָ������ǰ��״̬����������Һ����仯����
��4��pH��Ϊ4��A��Һ��D��Һ�У���ˮ�������c��H+��֮����_________��
��5��K��A�������Ӷ�H2O2�ֽ�Ĵ����ã�Ϊ�Ƚ�K��A��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺
��1����Ӧ�ڵĻ�ѧ����ʽ��____________ ��
��2����Ӧ�ٵ����ӷ���ʽ��_________________��
��3���ö��Ե缫���400.00mL A��Һ��һ��ʱ���ڲ����ҺpH��1������Ҫ����Һ�м���__________��������Ϊ______g������ʹ��Һ�ָ������ǰ��״̬����������Һ����仯����
��4��pH��Ϊ4��A��Һ��D��Һ�У���ˮ�������c��H+��֮����_________��
��5��K��A�������Ӷ�H2O2�ֽ�Ĵ����ã�Ϊ�Ƚ�K��A��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺
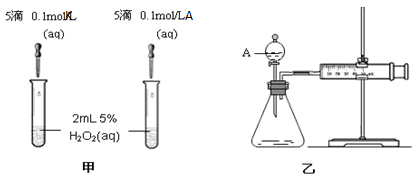
���Է�������ͼ��ͨ���۲����ݲ����Ŀ����ɾ����ԱȽϵó����ۡ���ͬѧ�����ʵ�鲻̫��������������__________________��
������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ__________��ʵ������Ҫ������������___________��
������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ__________��ʵ������Ҫ������������___________��
��1��2FeCl3 + Cu =2FeCl2 + CuCl2
��2��3Cu + 8H+ +2NO3- = 3Cu2+ + 2NO�� + 4H2O
��3��CuO��1.6
��4��106��1
��5������Һ�������Ӳ�ͬ��Ҳ���ܶ�H2O2�ķֽ������ͬ�����ã���Һ©�����ռ�40mL�����ʱ��
��2��3Cu + 8H+ +2NO3- = 3Cu2+ + 2NO�� + 4H2O
��3��CuO��1.6
��4��106��1
��5������Һ�������Ӳ�ͬ��Ҳ���ܶ�H2O2�ķֽ������ͬ�����ã���Һ©�����ռ�40mL�����ʱ��

��ϰ��ϵ�д�
�����Ŀ