��Ŀ����
����TiO2��Ϊһ�ֹ����Խ��Խ�ܵ����ǵĹ�ע�������㷺�������á�

(1)��ȡ����TiO2�ķ����ܶ࣬��������ˮ�ⷨ�ǽ�TiCl4���嵼������������(700~1000��)����ˮ�⣬�仯ѧ��ӦʽΪ_______________��
(2)���������ѿɹ��ӷ����л���Ⱦ��(VOCs)������ˮ �������ڣ�������ϩ���ⷴӦΪ��
C2HCl3 +2O2��2CO2+ HCl+Cl2�������㹻���Ľ�����β����ʵ���Ҽ���������������ļ�����
________________________��ͨ�������Ƿ��ֻ��ж��ָ��������֮һΪ�� ����л���˴Ź���������____���塣
����л���˴Ź���������____���塣
(3)���ð뵼����TiO2��Ⱦ�ϡ����缫��I3-��I-�Ļ����������ʣ�I2+I-=I-�����ɹ���Ⱦ������̫���ܵ�� (DSSCs)������ԭ����ͼ��С���õ�ع���ʱ�������ĵ缫��ӦΪ________________��
(4)�ڲ�ͬ�����壨��Ƭ����Ƭ���մɣ������Ʊ��������ѱ�Ĥ�������첻ͬ����TiO2��Ĥ���ʹ������ɫ��ÿ�ι���20minȡһ������ʵ��������ͼ��
(2)���������ѿɹ��ӷ����л���Ⱦ��(VOCs)������ˮ �������ڣ�������ϩ���ⷴӦΪ��
C2HCl3 +2O2��2CO2+ HCl+Cl2�������㹻���Ľ�����β����ʵ���Ҽ���������������ļ�����
________________________��ͨ�������Ƿ��ֻ��ж��ָ��������֮һΪ��
 ����л���˴Ź���������____���塣
����л���˴Ź���������____���塣 (3)���ð뵼����TiO2��Ⱦ�ϡ����缫��I3-��I-�Ļ����������ʣ�I2+I-=I-�����ɹ���Ⱦ������̫���ܵ�� (DSSCs)������ԭ����ͼ��С���õ�ع���ʱ�������ĵ缫��ӦΪ________________��
(4)�ڲ�ͬ�����壨��Ƭ����Ƭ���մɣ������Ʊ��������ѱ�Ĥ�������첻ͬ����TiO2��Ĥ���ʹ������ɫ��ÿ�ι���20minȡһ������ʵ��������ͼ��

����˵����ȷ����____��
a.��ͬ���壬���ۺ����¶�һ������Ƭ���
b.Լ��520��ʱ����Ƭ����Ĺ���������
c.���ۺ���TiO2�������������¶ȵ����߶�����
d.��ͬ����TiO2��Ĥ�Ĺ�����Բ�ͬ
a.��ͬ���壬���ۺ����¶�һ������Ƭ���
b.Լ��520��ʱ����Ƭ����Ĺ���������
c.���ۺ���TiO2�������������¶ȵ����߶�����
d.��ͬ����TiO2��Ĥ�Ĺ�����Բ�ͬ
(1)TiCl4+2H2+O2 TiO2+4HCl(��TiCl4+2H2O
TiO2+4HCl(��TiCl4+2H2O TiO2+4HCl)
TiO2+4HCl)
(2)��ʪ��ĵ⻯�ص�����ֽ���飻3
(3)I3-+2e-=3I-����I2+2e-=2I-��
(4)bd
 TiO2+4HCl(��TiCl4+2H2O
TiO2+4HCl(��TiCl4+2H2O TiO2+4HCl)
TiO2+4HCl) (2)��ʪ��ĵ⻯�ص�����ֽ���飻3
(3)I3-+2e-=3I-����I2+2e-=2I-��
(4)bd

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
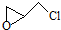 ������л���˴Ź���������
������л���˴Ź���������

 w ww.k s5u. co m
w ww.k s5u. co m
 C6H12O6�������ǣ�+6O2��
C6H12O6�������ǣ�+6O2��

 C6H12O6�������ǣ�+6O2��
C6H12O6�������ǣ�+6O2��