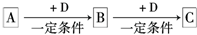��Ŀ����
��֪A��B��C�dz����ĵ��ʣ���һ���������ת���Ĺ�ϵ����ͼ��ʾ(�в�������ʡ��)��
(1)�������£�BΪ���壬AΪ��ɫ���壬CԪ������Ȼ���к���λ�ڵ�����EΪ��ɫ���壬д��C+E![]() D�Ļ�ѧ����ʽ��_______________��
D�Ļ�ѧ����ʽ��_______________��
(2)��������A��B��Ϊ���壬CΪ�������ʣ�DΪ��ɫ���壬д��C+E![]() D�Ļ�ѧ����ʽ��______________________________________________��
D�Ļ�ѧ����ʽ��______________________________________________��
(3)��������BΪ���壬AΪ��ɫ�ǽ������壬CΪ������д��C+E![]() D�Ļ�ѧ����ʽ��________________________________________________��
D�Ļ�ѧ����ʽ��________________________________________________��
(1)2Al+3CuO![]() 3Cu+Al2O3
3Cu+Al2O3
(2)3Fe+4H2O(g)![]() Fe3O4+4H2
Fe3O4+4H2
(3)2Mg+CO2![]() 2MgO+C
2MgO+C
������(1)CԪ������Ȼ���к���λ�ڵ�������֪CΪAl����AΪ��ɫ���壬BΪ���壬����ת����ϵͼ����֪AΪCu��BΪO2����EΪCuO��C+E![]() D�Ļ�ѧ����ʽΪ2Al+3CuO
D�Ļ�ѧ����ʽΪ2Al+3CuO![]() 3Cu+Al2O3
3Cu+Al2O3
(2)������A��B��CΪ�������ʣ�����A��BΪ���壬CΪ�������ʣ�DΪ��ɫ���壬�������ǵ��ת����ϵ����֪��AΪH2��BΪO2��CΪFe��DΪFe2O3������C+E![]() D�Ļ�ѧ����ʽΪ3Fe+4H2O(g)
D�Ļ�ѧ����ʽΪ3Fe+4H2O(g)![]() Fe3O4+4H2��
Fe3O4+4H2��
(3)�����£�AΪ��ɫ�ǽ������壬����֪AΪ̼���ʣ�BΪ���壬��BΪO2��EΪCO2��CΪ����Mg��C+E![]() D��Ϊ2Mg+CO2
D��Ϊ2Mg+CO2![]() 2MgO+C��
2MgO+C��