��Ŀ����
��8�֣��ó������ⶨNaHCO3��K2CO3��������ɡ�ʵ�������ÿ�γ�ȡһ����������Ʒ����ˮ�Ƴ���Һ�������еμ���ͬŨ�ȵ�Ba(OH)2��Һ500mL��ÿ��ʵ�����ַ�Ӧ��ʵ���¼���£�
�ش��������⣺
(1)��2��ʵ���в������������� ��
(2)Ba(OH)2��Һ�����ʵ����ʵ���Ũ���� ��
(3)��2��ʵ����Ʒ��NaHCO3�����ʵ����� ��
�����Ͼ�Ҫ��д�������̣�
| ʵ����� | ��Ʒ����/g | ��������/g |
| 1 | 1.716 | 2.758 |
| 2 | 2.574 | |
| 3 | 3.432 | 5.516 |
| 4 | 4.290 | 5.516 |
(1)��2��ʵ���в������������� ��
(2)Ba(OH)2��Һ�����ʵ����ʵ���Ũ���� ��
(3)��2��ʵ����Ʒ��NaHCO3�����ʵ����� ��
�����Ͼ�Ҫ��д�������̣�
��8�֣�(1)4.137 ��2�֣� (2)0.056mol/L ��3�֣� (3)0.006mol ��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ��̼���ƣ�Na2CO3��10H2O������ g��
��̼���ƣ�Na2CO3��10H2O������ g��
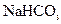 ��������ȡ�����Ҫװ������:
��������ȡ�����Ҫװ������: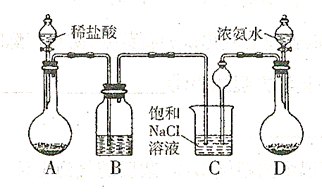
 ,�����D������(ֻ��һ�� ��Cװ��������©���������� ��
,�����D������(ֻ��һ�� ��Cװ��������©���������� ��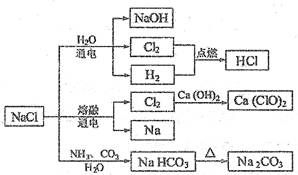

 ol��L��1Na2CO3��Һ�����в�����ȷ���� ( )
ol��L��1Na2CO3��Һ�����в�����ȷ���� ( )