��Ŀ����
����Ŀ����1���ڢ�![]() ��ʯī��C60��
��ʯī��C60��![]() ��CH3CH2OH��
��CH3CH2OH��![]() ��
��![]() ��CH3OCH3�У���Ϊͬλ�أ�______��Ϊͬ���칹��_______����Ϊͬ��������_______������ţ���
��CH3OCH3�У���Ϊͬλ�أ�______��Ϊͬ���칹��_______����Ϊͬ��������_______������ţ���
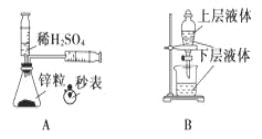
��2�����Т�CaCl2 �ڽ��ʯ ��NH4Cl ��Na2SO4 �ݱ����������ʣ�������Ҫ��ش�
I.�ۻ�ʱ����Ҫ�ƻ���ѧ������_______���۵���ߵ���_________��������ţ�
II.ֻ�������Ӽ���������_________�������Է��Ӽ���������ϵ���_________��������ţ�
��3��д���������ʵĵ���ʽ
��H2O______��NaOH______��NH3______��
��4��д��CO2�Ľṹʽ______��д����ԭ�ӵ�ԭ�ӽṹʾ��ͼ______��
���𰸡��٢� �ݢ� �ڢ� �� �� �� �� ![]()
![]()
 O=C=O
O=C=O 
��������
��1����ͬλ�أ���������ͬ����������ͬ��ͬһԪ�صIJ�ͬԭ�ӣ�ͬ���칹�壺����ʽ��ͬ���ṹ��ͬ�����ʣ�ͬ�������壺ͬ��Ԫ���γɵIJ�ͬ���ʣ����ݸ�����������
��2����ȷ���������ͣ���ȷ����ѧ����
��3����ȷ����ѧ�����ͣ���ȷ������ʽ��
��4����ȷ����ѧ�����ͣ���ȷ���ṹʽ����ԭ��Ϊ16��Ԫ�أ�д��ԭ�ӽṹʾ��ͼ��
��1������ͬλ�أ���������ͬ����������ͬ��ͬһԪ�صIJ�ͬԭ�ӣ�ͬ���칹�壺����ʽ��ͬ���ṹ��ͬ�����ʣ�ͬ�������壺ͬ��Ԫ���γɵIJ�ͬ���ʡ���ˢ٢�Ϊͬλ�أ��ݢΪͬ���칹�壻�ڢۻ�Ϊͬ�������壻
�𰸣��٢� �ݢ� �ڢ�
��2����CaCl2Ϊ���Ӿ��壬�������Ӽ����ڽ��ʯΪԭ�Ӿ��壬���ڹ��ۼ��� ��NH4Cl ���Ӿ��壬�������Ӽ����ۼ�����Na2SO4Ϊ���Ӿ��壬�������Ӽ����ۼ��� �ݱ�Ϊ���Ӿ��壬���ڹ��ۼ���
I.�ۻ�ʱ���Ӿ����ƻ����Ӽ���ԭ�Ӿ����ƻ����ۼ������Ӿ����ƻ����Ӽ���������ͨ��������۵�ԭ�Ӿ���>���Ӿ���>���Ӿ��壻
II.ֻ�������Ӽ����������Ȼ��ƣ����Ӿ����Է��Ӽ���������ϣ�
�𰸣��� �� �� ��
��3����H2OΪ���ۻ��������O-H���ۼ���NaOH�������Ӻ����������Ӵ������Ӽ��������������ڲ�����O-H���ۼ��۰���Ϊ���ۻ��������N-H���ۼ���
�����ʽΪ![]() ��
�� ![]() ��
��  ��
��
�𰸣�![]()
![]()

��4��CO2Ϊ���ۻ��������8�����ȶ��ṹ��ȷ���ṹʽΪO=C=O����ԭ��Ϊ16��Ԫ�أ�ԭ�ӽṹʾ��ͼ ��
��
�𰸣�O=C=O 

����Ŀ���������״���;����㷺��Խ��Խ�����̼ҵĹ�ע����ҵ�ϼ״��ĺϳ�;�����ֶ���������ʵ������ģ��״��ϳɷ�Ӧ,��2 L�ܱ������ڣ�400 ��ʱ������ӦCO(g)+2H2(g) ![]() CH3OH(g)����ϵ��n(CO)��ʱ��ı仯�����
CH3OH(g)����ϵ��n(CO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 5 |
n(CO)(mol) | 0.020 | 0.011 | 0.008 | 0.007 | 0.007 |

��1��ͼ�б�ʾCH3OH �ı仯��������_______��
��2�����д�ʩ������߷�Ӧ���ʵ���_________(������Ӧ��ĸ���)��
a.�����¶� b.������� c.����ѹǿ d.��ʱ�����CH3OH
��3������������˵����Ӧ�ﵽƽ��״̬����__________(������Ӧ��ĸ���)��
a.CO��H2��Ũ�ȱ��ֲ��� b.v(H2)=2 v(CO)
c.CO�����ʵ����������ֲ��� d.�����������ܶȱ��ֲ���
e.ÿ����1molCH3OH��ͬʱ��2molH-H������
��4��CH3OH��O2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ����ͼ��ʾ��ͼ��CH3OH��__________����A��B��ͨ�룬b���ĵ缫��Ӧʽ��__________��