��Ŀ����
���Ȼ�������������ɫҺ�壬�ڿ����м���ˮ�⣬�۵�
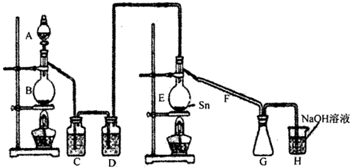
(1)�ò�����(δ����)��������װ�ã���ȷ��˳����(����ӿڵĴ�����ĸ)( )��( )��( )��( )��( )��( )��( )��( )��( )��( )��?
(2)װ�â���������__________________��װ�â���������________________________��
(3)����������ȴˮ�������Ǵ�_____________���룬��_____________������
(4)ʵ��ʱӦ�ȵ�ȼ_____________���ƾ��ƣ������¶�Ӧ����_____________�棬��_____________����ֹͣ���ȡ�
(5)��֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ________________________��
(6)�������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ�����������____________________��
(7)Ϊ�˷�ֹ��Ⱦ��������װ�õ����Ӧ__________________________________________��
��1��B I J K A C D H G E��F
��2����ȥCl2�е�HCl��ˮ���� ��ֹ������ˮ���������ռ�SnCl4��ƿ��
��3��Q P
��4��I 231 �����ۻ�
��5��SnCl4+2H2O![]() SnO2+4HCl��
SnO2+4HCl��
��6���а�ɫ��������
��7����ʢ��NaOH��Һ���ձ����ն���Cl2
����������Ҫ��ɵķ�Ӧ�ǣ�Sn(��)+2Cl2(g)![]() SnCl4��ʵ�����Ϊ����Sn�ۻ������Ʊ����﴿����Cl2���Ҹ�����Ŀ����ʾ��Ҫ��ֹSnCl4��ˮ��ͼ���������Ӧ�����ڷų�������ֹͣ�������ȡ���Ŀ������6��װ������Ϊ����.��Ӧװ�ã���.����װ�ã���.����װ�ã���.����װ�ã���.����װ�ã���.����װ�á��ɴ˿��Իش����Ӵ�����������������Ӧ�����������ա�����������������������������������ע����ȷ�ӿڡ�
SnCl4��ʵ�����Ϊ����Sn�ۻ������Ʊ����﴿����Cl2���Ҹ�����Ŀ����ʾ��Ҫ��ֹSnCl4��ˮ��ͼ���������Ӧ�����ڷų�������ֹͣ�������ȡ���Ŀ������6��װ������Ϊ����.��Ӧװ�ã���.����װ�ã���.����װ�ã���.����װ�ã���.����װ�ã���.����װ�á��ɴ˿��Իش����Ӵ�����������������Ӧ�����������ա�����������������������������������ע����ȷ�ӿڡ�