��Ŀ����
(1998��ȫ����35)�������������ڲ�ͬ�¶��µ��ܽ��(g/100 g H2O)
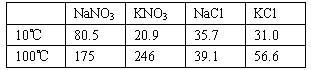
(����ʱ�ٶ����������ʱ��Ӱ����Ե��ܽ�ȣ��ڹ��˾���ʱ���ܼ���ĺ��Բ��ơ�)
(1)ȡ23.4 g NaCl��40.4 g KNO3����70.0 g H2O�������ܽ⡣��100��ʱ������50.0 g H2O��ά�ָ��¶ȣ������������壬�������þ��������(![]() )��
)��
����Һ��ȴ��10�棬����ֽᾧ���ˡ��������þ��������(![]() )��
)��
(2)��ȡ34.0 g NaNO3��29.8 g KCl��ͬ����������ʵ�顣10��ʱ�����ľ����� (д��ѧʽ)��100���10��õ��ľ�������(m��������m������)�ֱ��Ƕ���?
�𰸣�
(1)15.6 g 36.9 g (2)KNO3��NaCl 15.6 g 36.9 g

��ϰ��ϵ�д�
�����Ŀ


