��Ŀ����
�����������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ���£�
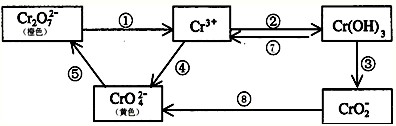
�ش��������⣺
��1������ת����ϵ������������ԭ��Ӧ����______�����ţ���������Ҫʹ������������______�����ţ���
��2����Ӧ���ǿ��淴Ӧ����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��д���÷�Ӧ�����ӷ���ʽ��______��
��3����ͼʾ��Ϣ��֪����һ�ֺ��������������ԣ�д���û�������NaOH��Һ��Ӧ�����ӷ���ʽ��______��
��4����ҵ�ϴ�����Cr2O72-�ķ�ˮʱ��һ�㽫�綾��Cr2O72-ת��ΪCr3+����̼Ϊ����������������������NaCl��Cr2O72-�����Է�ˮ��д���缫��Ӧ����Һ�н��еķ�Ӧ�����ӷ���ʽ������______������______��Һ��______��
��5����֪Ag2CrO4��AgCl��Ksp�ֱ�Ϊ9.0��10-12��1.56��10-10��������ͬŨ�ȵ�Na2CrO4��NaCl�Ļ����Һ����μ�����������Һ���������ɵij�����______��

�ش��������⣺
��1������ת����ϵ������������ԭ��Ӧ����______�����ţ���������Ҫʹ������������______�����ţ���
��2����Ӧ���ǿ��淴Ӧ����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��д���÷�Ӧ�����ӷ���ʽ��______��
��3����ͼʾ��Ϣ��֪����һ�ֺ��������������ԣ�д���û�������NaOH��Һ��Ӧ�����ӷ���ʽ��______��
��4����ҵ�ϴ�����Cr2O72-�ķ�ˮʱ��һ�㽫�綾��Cr2O72-ת��ΪCr3+����̼Ϊ����������������������NaCl��Cr2O72-�����Է�ˮ��д���缫��Ӧ����Һ�н��еķ�Ӧ�����ӷ���ʽ������______������______��Һ��______��
��5����֪Ag2CrO4��AgCl��Ksp�ֱ�Ϊ9.0��10-12��1.56��10-10��������ͬŨ�ȵ�Na2CrO4��NaCl�Ļ����Һ����μ�����������Һ���������ɵij�����______��
��1����������ת���У�����CrԪ�صĻ��ϼ۽��ͣ��ܢ���CrԪ�صĻ��ϼ����ߣ���٢ܢ�����������ԭ��Ӧ���Ңܢ�����Ҫ������������
�ʴ�Ϊ���٢ܢࣻ�ܢࣻ
��2����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��ת���ݿ�֪�����������ӷ�ӦΪ2CrO42-+2H+

Cr2O72-+H2O��
�ʴ�Ϊ��2CrO42-+2H+

Cr2O72-+H2O��
��3��ת��ͼ��ֻ�����������������������ƣ������������������ԣ�����Ӧ�����ӷ�ӦΪCr��OH��3+OH-=CrO2-+2H2O��
�ʴ�Ϊ��Cr��OH��3+OH-=CrO2-+2H2O��
��4��������������������ӦΪFe-2e=Fe2+����Һ�е�������H+�������ŵ磬��������ӦΪH++2e=H2�������������������Ӿ��л�ԭ�ԣ���
Cr2O72-����������ԭ��Ӧ��Cr2O72-ת��ΪCr3+������Һ�з��������ӷ�ӦΪ6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O��
�ʴ�Ϊ��Fe-2e=Fe2+��H++2e=H2����6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O������
��5��Ag2CrO4��AgCl��Ksp�ֱ�Ϊ9.0��10-12��1.56��10-10��������ͬŨ�ȵ�Na2CrO4��NaCl�Ļ����Һ����μ�����������Һ��
��Na2CrO4��NaCl��Ũ�ȶ�Ϊ1mol/L��
Ag2CrO4����ʱ��Ҫ��c��Ag+��=
=3��10-6��
AgCl����ʱ��Ҫ��c��Ag+��=1.56��10-10����AgCl�ȳ������ʴ�Ϊ��AgCl��
�ʴ�Ϊ���٢ܢࣻ�ܢࣻ
��2����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��ת���ݿ�֪�����������ӷ�ӦΪ2CrO42-+2H+

Cr2O72-+H2O��
�ʴ�Ϊ��2CrO42-+2H+

Cr2O72-+H2O��
��3��ת��ͼ��ֻ�����������������������ƣ������������������ԣ�����Ӧ�����ӷ�ӦΪCr��OH��3+OH-=CrO2-+2H2O��
�ʴ�Ϊ��Cr��OH��3+OH-=CrO2-+2H2O��
��4��������������������ӦΪFe-2e=Fe2+����Һ�е�������H+�������ŵ磬��������ӦΪH++2e=H2�������������������Ӿ��л�ԭ�ԣ���
Cr2O72-����������ԭ��Ӧ��Cr2O72-ת��ΪCr3+������Һ�з��������ӷ�ӦΪ6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O��
�ʴ�Ϊ��Fe-2e=Fe2+��H++2e=H2����6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O������
��5��Ag2CrO4��AgCl��Ksp�ֱ�Ϊ9.0��10-12��1.56��10-10��������ͬŨ�ȵ�Na2CrO4��NaCl�Ļ����Һ����μ�����������Һ��
��Na2CrO4��NaCl��Ũ�ȶ�Ϊ1mol/L��
Ag2CrO4����ʱ��Ҫ��c��Ag+��=
| 9.0��10-12 |
AgCl����ʱ��Ҫ��c��Ag+��=1.56��10-10����AgCl�ȳ������ʴ�Ϊ��AgCl��

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ

 Cr2O72-+H2O
Cr2O72-+H2O ��2010?��ͷһģ�������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ����
��2010?��ͷһģ�������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ���� Cr2O72-+H2O
Cr2O72-+H2O
 Cr2O72-+H2O
Cr2O72-+H2O
