��Ŀ����
ij�������
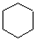
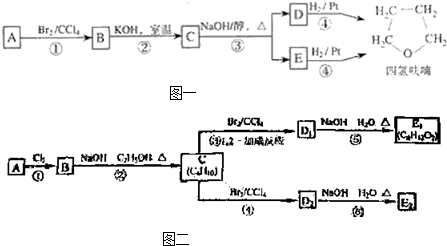
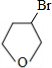
A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź������ױ���������ֻ��һ�����͵��⣬��A��������ͼ��ʾ��ת����ϵ������D1��D2��Ϊͬ���칹�壬E1��E2��Ϊͬ���칹�壮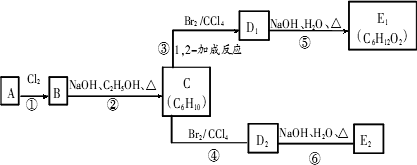
(1)д���������ʵĽṹ��ʽ��
A________��C________��E2________��
(2)��ϵͳ����������C����________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ������������ָ����Ӧ�ķ�Ӧ���ͣ�
��Ӧ�ڣ�________(����)��
��Ӧ�ܣ�________(����)��
��Ӧ�ޣ�________(����)��
�𰸣�

��ϰ��ϵ�д�
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
�����Ŀ
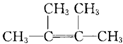

 +2NaOH
+2NaOH +2NaCl+2H2O
+2NaCl+2H2O
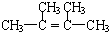




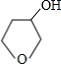 +NaBr
+NaBr