��Ŀ����
 ԭ���������������A��B��C��D��E���ֶ�����Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�B�ĵ��ʷ����������Թ��õ��ӣ�C�����������Ǵ�����������3����A��Dͬ���壬Eԭ�ӵ�������Cԭ�ӵ�������5������ش��������⣺
ԭ���������������A��B��C��D��E���ֶ�����Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�B�ĵ��ʷ����������Թ��õ��ӣ�C�����������Ǵ�����������3����A��Dͬ���壬Eԭ�ӵ�������Cԭ�ӵ�������5������ش��������⣺��1��A��C��D�γɵĻ�����W�ĵ���ʽ
��2����֪B2A4��BA3�������Ƶ����ʣ�B2A4ͨ�����Ⱥ�ɫ�ķ�ĩ����ɫ�ķ�ĩ��Ϊ��ɫ���Ҳ���Դ�������Ⱦ���仯ѧ��Ӧ����ʽ
��3��A2��B2�ڹ�ҵ�Ͽɺϳ�һ����Ҫ��ԭ��BA3��
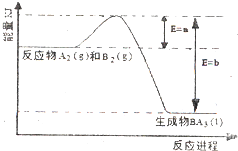
����ͼ��һ���¶Ⱥ�ѹǿ��A2��g����B2��g����Ӧ����1molBA3��l�������������仯ʾ��ͼ����֪��BA3��l��?BA3��g����H=+ckJ/mol����д����ҵ�ϳ�BA3��g�����Ȼ�ѧ��Ӧ����ʽ
��25��ʱ��x mol?L-1��BA3ˮ��Һ��pH=14-n��������ˮ�ĵ��룬��BA3ˮ�Ϸ��ӵĵ���ƽ�ⳣ��
��4��������ס��Ҷ���A��B��C����Ԫ����ɣ���Ϊ�����Ϊǿ������£�������Һ��ˮ�ĵ���̶���ͬ��
��������Һ��pH=a�������Һ��ˮ���������c��H+��=
������Һ�������Ϻ����Һ�е�����Ũ���ɴ�С����
��5����֪A��B��C����Ԫ����ɵ�һ�����ӻ�����B2A4C2������Һ�����ԣ�ԭ��Ϊ
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������A��B��C��D��EΪ���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶��С����AΪ�⣻BԪ�صĵ����к������Թ��õ��Ӷԣ���BΪ����CԪ��ԭ�ӵ������������Ǵ�����������3��������������������8������C�����6�������Ϊ2��CΪ��Ԫ�أ�A��Dͬ���壬D��ԭ����������O����DΪNa��
Eԭ�ӵ�������Cԭ�ӵ�������5������EΪ�����ݴ˷������1����2��С�⼴�ɣ�
��3�������Ȼ�ѧ��Ӧ����ʽ���ȼ��㣬����д��
��4���پ�ˮ�����ӻ���������ʽKw=C��H+��?c��OH-������������Һ��ˮ�������������Ũ�ȣ�
��������У�笠�����ˮ�����Һ��ʾ���ԣ���������Ũ�ȴ�С�Ƚ�֪ʶ���жϣ�
��5����������ˮ��ԭ���شɣ�
Eԭ�ӵ�������Cԭ�ӵ�������5������EΪ�����ݴ˷������1����2��С�⼴�ɣ�
��3�������Ȼ�ѧ��Ӧ����ʽ���ȼ��㣬����д��
��4���پ�ˮ�����ӻ���������ʽKw=C��H+��?c��OH-������������Һ��ˮ�������������Ũ�ȣ�
��������У�笠�����ˮ�����Һ��ʾ���ԣ���������Ũ�ȴ�С�Ƚ�֪ʶ���жϣ�
��5����������ˮ��ԭ���شɣ�
���
�⣺���ݷ�����֪��AΪ�⣬BΪ����CΪ����DΪ�ƣ�EΪ����
��1��H��O��Na�γɵĻ�����WΪ�������ƣ��������Ƶĵ���ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��N2H4��NH3�������Ƶ����ʣ�N2H4ͨ�����Ⱥ�ɫ�ķ�ĩ����ͭ������ͭ�ĺ�ɫ�ķ�ĩ��Ϊ��ɫͭ���ʣ��Ҳ���Դ�������Ⱦ�����嵪�����仯ѧ��Ӧ����ʽΪ��N2H4+2CuO
2Cu+N2��+2H2O���ʴ�Ϊ��N2H4+2CuO
2Cu+N2��+2H2O��
��3������ͼ��֪��N2��H2��Ӧ����1molNH3��l���ų�������Ϊ��b-a��kJ��NH3��l��?NH3��g����H=+ckJ/mol����N2��g��+3H2��g��?2NH3��g���ķ�Ӧ���Ȼ�ѧ��Ӧ����ʽΪN2��g��+3H2��g��?2NH3��g����H=-2��b-a-c��kJ?mol-1��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-2��b-a-c��kJ?mol-1��
��x mol?L-1��NH3ˮ��Һ��pH=14-n������Һ��c��H+��=10-��14-n��=10n-14����c��OH-��=
=10-n��K=
=
��
�ʴ�Ϊ��
��
��4��������ס��Ҷ���H��N��O����Ԫ����ɣ���Ϊ���ΪNH3?H2O����Ϊǿ�ᣬ��ΪHNO3��
����������Һ��pH=a����Kw=C��H+��?c��OH-��������Һ��ˮ���������C��H+��=c��OH-��=
=10a-14mol/L���ʴ�Ϊ��10a-14mol/L��
������Һ�������Ϻ�����Ӧ��������泥�笠�����ˮ�����Һ��ʾ���ԣ�������Һ�е�����Ũ���ɴ�С��˳����c��NH4+����c��NO3-����c��OH-����c��H+�����ʴ�Ϊ��c��NH4+����c��NO3-����c��OH-����c��H+����
��5��H��N��O����Ԫ����ɵ�һ�����ӻ�����N2H4O2ΪNH4NO2����Ϊǿ�������Σ�NH4+��ˮ��̶ȴ���NO2-��ˮ��̶ȣ���ʹ����Һ�����ԣ��ʴ�Ϊ��NH4+��ˮ��̶ȴ���NO2-��ˮ��̶ȣ�
��1��H��O��Na�γɵĻ�����WΪ�������ƣ��������Ƶĵ���ʽΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��N2H4��NH3�������Ƶ����ʣ�N2H4ͨ�����Ⱥ�ɫ�ķ�ĩ����ͭ������ͭ�ĺ�ɫ�ķ�ĩ��Ϊ��ɫͭ���ʣ��Ҳ���Դ�������Ⱦ�����嵪�����仯ѧ��Ӧ����ʽΪ��N2H4+2CuO
| ||
| ||
��3������ͼ��֪��N2��H2��Ӧ����1molNH3��l���ų�������Ϊ��b-a��kJ��NH3��l��?NH3��g����H=+ckJ/mol����N2��g��+3H2��g��?2NH3��g���ķ�Ӧ���Ȼ�ѧ��Ӧ����ʽΪN2��g��+3H2��g��?2NH3��g����H=-2��b-a-c��kJ?mol-1��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-2��b-a-c��kJ?mol-1��
��x mol?L-1��NH3ˮ��Һ��pH=14-n������Һ��c��H+��=10-��14-n��=10n-14����c��OH-��=
| 10-14 |
| 10n-14 |
| c(OH-)��c(NH4+) |
| c(NH3?H2O) |
| 10-2n |
| x-10-n |
�ʴ�Ϊ��
| 10-2n |
| x-10-n |
��4��������ס��Ҷ���H��N��O����Ԫ����ɣ���Ϊ���ΪNH3?H2O����Ϊǿ�ᣬ��ΪHNO3��
����������Һ��pH=a����Kw=C��H+��?c��OH-��������Һ��ˮ���������C��H+��=c��OH-��=
| KW |
| 10-a |
������Һ�������Ϻ�����Ӧ��������泥�笠�����ˮ�����Һ��ʾ���ԣ�������Һ�е�����Ũ���ɴ�С��˳����c��NH4+����c��NO3-����c��OH-����c��H+�����ʴ�Ϊ��c��NH4+����c��NO3-����c��OH-����c��H+����
��5��H��N��O����Ԫ����ɵ�һ�����ӻ�����N2H4O2ΪNH4NO2����Ϊǿ�������Σ�NH4+��ˮ��̶ȴ���NO2-��ˮ��̶ȣ���ʹ����Һ�����ԣ��ʴ�Ϊ��NH4+��ˮ��̶ȴ���NO2-��ˮ��̶ȣ�
������������һ������ѧ����ѧ�ۺ�֪ʶ����Ŀ��Ҫ��ѧ�����з����ͽ��������������Ѷȴ�

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A�����ʵ���Ũ�Ⱥ��������ͬ������ʹ�����Һ����������п��Ӧʱ����ʼʱ���߲���H2���ʻ������ |
| B��100 mL 1 mol?L-1�������50 mL 2 mol?L-1�����ᣬ�ֱ���������п��Ӧʱ�����߷ų�H2���ʺ���������� |
| C��100 mL pH=3��H2SO4��HCl��Һ��������п��Ӧ�ų�H2��������� |
| D��100 mL pH=3�������������Һ��������п��Ӧ������H2��������� |
��ȡ10mL��ı���ˮ��Һ�������Һ©���У�Ȼ��ע��4mL���Ȼ�̼���������ã�ʵ������Ϊ��������
| A��Һ��ֲ㣬�ϲ�Ϊ���Ȼ�̼�㣬��ɫ |
| B��Һ��ֲ㣬�ϲ�Ϊˮ�㣬��ɫ |
| C��Һ��ֲ㣬�²�Ϊ���Ȼ�̼�㣬��ɫ |
| D��Һ��ֲ㣬�²�Ϊˮ�㣬��ɫ |

 +R
+R +R
+R
