��Ŀ����
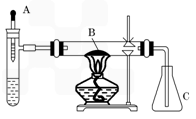
��֪�����ؽ����������ܴ�H2O2�ķֽ⡣ͼ1-4�ǰ��Ĵ�������ʵ��װ�á��Թ��м������Ũ��ˮ������2mL5��AgNO3��Һ��AΪ��ͷ�ιܣ�Ԥ������25����H2O2��Һ��B��װ�в�ʯ��������ȼ�ƾ���һ��ʱ���A�е�H2O2��Һ�����ص����Թܣ��Թ��ڲ����������塣���ã���ƿC�ڳ��ֺ���ɫ���塣�ش��������⣺
ͼ1-4
(1)����Ϊ��Ӧ���ܴ��ֽ�H2O2��������(д��ѧʽ)__________________��
(2)�Թ��в�����������_________��
(3)B�з�����Ӧ�Ļ�ѧ����ʽΪ__________________��
(4)��ʵ�黹���Ը�������ҩƷ���������Թ��мӹ���__________________����ͷ�ι�Ԥ������Һ��_________��
(5)������װ���⣬��ʵ��װ�õ�ȱ���ǻ�ȱ��_________װ�á�
(1)[Ag(NH3)2]+{��[Ag(NH3)2]OH��[Ag(NH3)2]NO3}
(2)O2 ��NH3
(3)4NH3+5O2![]() 4NO+6H2O
4NO+6H2O
(4) Na2O2 Ũ��ˮ
(5)�����
��ʾ��Ũ��ˮ����������Ӧ�ɲ���������Һ��������������Һ�е�Ag(NH3)2+������á��Թ�A������B�е�����ض�����������������ˮ�����ȳɷ֡�Bװ���з�������������Ӧ�����Թ�A�з���������Ƶ����ʣ���ͷ�ι���װŨ��ˮ����Ũ��ˮ����������ƹ�����Ҳ�ܷų����������������塣���ڹ�������ֽ��Ƿ��ȷ�Ӧ������A�зų��������л��бȽ϶��ˮ������ˮ�������ڻ�Ӱ�찱�Ĵ����������������A��B֮������һ����ˮ����װ�á���Ҫ����Ԫ�ػ������ѧʵ����ơ���ѧʵ������Ĺ۲�������

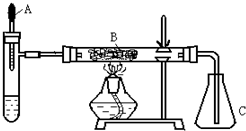 ��֪�����ؽ����������ܴ�H2O2�ķֽ⣮�Թ��м������Ũ��ˮ������2ml 5%AgNO3��Һ��AΪ��ͷ�ιܣ�Ԥ������25%��H2O2��Һ�� B��װ�в�ʯ��������ȼ�ƾ���һ��ʱ���A�е�H2O2��Һ�����ص����Թܣ��Թ��ڲ����������壮���ã���ƿC�ڳ������Ե���ɫ�仯���ش��������⣺
��֪�����ؽ����������ܴ�H2O2�ķֽ⣮�Թ��м������Ũ��ˮ������2ml 5%AgNO3��Һ��AΪ��ͷ�ιܣ�Ԥ������25%��H2O2��Һ�� B��װ�в�ʯ��������ȼ�ƾ���һ��ʱ���A�е�H2O2��Һ�����ص����Թܣ��Թ��ڲ����������壮���ã���ƿC�ڳ������Ե���ɫ�仯���ش��������⣺
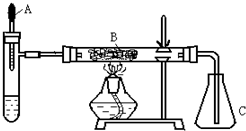 ��֪�����ؽ����������ܴ�H2O2�ķֽ⣮�Թ��м������Ũ��ˮ������2ml 5%AgNO3��Һ��AΪ��ͷ�ιܣ�Ԥ������25%��H2O2��Һ�� B��װ�в�ʯ��������ȼ�ƾ���һ��ʱ���A�е�H2O2��Һ�����ص����Թܣ��Թ��ڲ����������壮���ã���ƿC�ڳ������Ե���ɫ�仯���ش��������⣺
��֪�����ؽ����������ܴ�H2O2�ķֽ⣮�Թ��м������Ũ��ˮ������2ml 5%AgNO3��Һ��AΪ��ͷ�ιܣ�Ԥ������25%��H2O2��Һ�� B��װ�в�ʯ��������ȼ�ƾ���һ��ʱ���A�е�H2O2��Һ�����ص����Թܣ��Թ��ڲ����������壮���ã���ƿC�ڳ������Ե���ɫ�仯���ش��������⣺