��Ŀ����
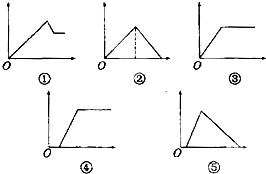 ����ͼ���У�������Ϊ����������ʵ�����������Ϊij��Һ�м��뷴Ӧ������ʵ����������⽫��Ӧ��ͼ������루1��-��5�����Ӧ��˳���ǣ�������
����ͼ���У�������Ϊ����������ʵ�����������Ϊij��Һ�м��뷴Ӧ������ʵ����������⽫��Ӧ��ͼ������루1��-��5�����Ӧ��˳���ǣ�������| ��Һ | �������� |
| ��1������ʯ��ˮ | ͨ�����CO2���� |
| ��2���Ȼ�����Һ | ���������ˮ |
| ��3��MgCl2��AlCl3�Ļ��Һ | ��μ���NaOH��Һ������ |
| ��4��������NaOH��NaAlO2��Һ | ��μ���ϡ���� |
| ��5��������NaOH��NaAlO2��Һ | ͨ�����CO2���� |
| A���٢ۢڢܢ� |
| B���ۢݢܢڢ� |
| C���٢ڢۢܢ� |
| D���ڢۢ٢ݢ� |
���㣺þ��������Ҫ������,���ӷ���ʽ����д
ר�⣺
��������ʾͼ���У�������Ϊ����������ʵ�����������Ϊij��Һ�м��뷴Ӧ������ʵ���
�ٱ�ʾ����ij��Һ���ȳ��������������������ܽ⣻
�ڱ�ʾ�Ⱥ����������ij��Һ���ȳ���������������ȫ���ܽ⣻
�۱�ʾ����ij��Һ�����������������ij��Һ�������������ӻ��ܽ⣻
�ܱ�ʾ����ij��Һ������һ��ʱ��ų��ֳ��������������������ij��Һ�������������ӻ��ܽ⣻
�ݱ�ʾ����ij��Һ������һ��ʱ��ų��ֳ��������������������ij��Һ������ȫ���ܽ⣮
�ٱ�ʾ����ij��Һ���ȳ��������������������ܽ⣻
�ڱ�ʾ�Ⱥ����������ij��Һ���ȳ���������������ȫ���ܽ⣻
�۱�ʾ����ij��Һ�����������������ij��Һ�������������ӻ��ܽ⣻
�ܱ�ʾ����ij��Һ������һ��ʱ��ų��ֳ��������������������ij��Һ�������������ӻ��ܽ⣻
�ݱ�ʾ����ij��Һ������һ��ʱ��ų��ֳ��������������������ij��Һ������ȫ���ܽ⣮
���
�⣺��1������ʯ��ˮͨ����CO2���壬Ӧ���г�����Ca��OH��2+CO2CO2=CaCO3��+H2O����ͨ��ǰһ��������CO2���壬�����ܽ⣺CaCO3+CO2+H2O=Ca��HCO3��2������ͼ��ӦΪ�ڣ�
��2����ˮ����������ܽ������������Ȼ�����Һ���������ˮ�ķ�Ӧ����ʽΪ��AlCl3+3NH3?H2O�TAl��OH��3��+3NH4Cl������ͼ��ӦΪ�ۣ�
��3��MgCl2��AlCl3�Ļ��Һ����μ���NaOH��Һ����������������������þ����������������MgCl2+2NaOH�TMg��OH��2��+2NaCl��AlCl3+3NaOH�TAl��OH��3��+3NaCl���������������ܽ⣬Al��OH��3 +NaOH�TNaAlO2+2H2O������ͼ��ӦΪ�٣�
��4��������NaOH��NaAlO2��Һ����μ���ϡ���ᣬ����NaOH��HCl�����кͷ�Ӧ��NaOH+HCl=NaCl+H2O������NaAlO2+HCl+H2O=Al��OH��3��+NaCl��Al��OH��3 +3HCl=AlCl3+3H2O������ͼ��ӦΪ�ݣ�
��5��������NaOH��NaAlO2��Һ��ͨ������CO2���壮����NaOH��CO2���巴Ӧ����ӦΪ��CO2+NaOH=NaHCO3��������NaAlO2+CO2+2H2O=NaHCO3 +Al��OH��3��������ͼ��ӦΪ�ܣ�
��ѡD��
��2����ˮ����������ܽ������������Ȼ�����Һ���������ˮ�ķ�Ӧ����ʽΪ��AlCl3+3NH3?H2O�TAl��OH��3��+3NH4Cl������ͼ��ӦΪ�ۣ�
��3��MgCl2��AlCl3�Ļ��Һ����μ���NaOH��Һ����������������������þ����������������MgCl2+2NaOH�TMg��OH��2��+2NaCl��AlCl3+3NaOH�TAl��OH��3��+3NaCl���������������ܽ⣬Al��OH��3 +NaOH�TNaAlO2+2H2O������ͼ��ӦΪ�٣�
��4��������NaOH��NaAlO2��Һ����μ���ϡ���ᣬ����NaOH��HCl�����кͷ�Ӧ��NaOH+HCl=NaCl+H2O������NaAlO2+HCl+H2O=Al��OH��3��+NaCl��Al��OH��3 +3HCl=AlCl3+3H2O������ͼ��ӦΪ�ݣ�
��5��������NaOH��NaAlO2��Һ��ͨ������CO2���壮����NaOH��CO2���巴Ӧ����ӦΪ��CO2+NaOH=NaHCO3��������NaAlO2+CO2+2H2O=NaHCO3 +Al��OH��3��������ͼ��ӦΪ�ܣ�
��ѡD��
���������⿼�黯ѧ��Ӧ��ͼ��Ĺ�ϵ�������ؼ�����������ط�Ӧ����������д����Ӧ�ķ�Ӧ�ķ���ʽ�������ҳ���Ӧ��ͼ�μ��ɽ��⣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
һ�������£����Ϊ5L���ܱ������У�1molX��1molY���з�Ӧ��2X��g��+Y��g��Z��g������60s�ﵽƽ�⣬����0.3molZ������˵����ȷ���ǣ�������
A�������������Ϊ10L��Z��ƽ��Ũ�ȱ�Ϊԭ����
| ||
| B����XŨ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.001mol/��L?S�� | ||
| C���������¶ȣ�X���������������÷�Ӧ�ġ�H��0 | ||
| D��������ѹǿ��������Y��ת�������� |
���г�ȥ���ʣ������ڵ����ʣ�������ȷ���ǣ�������
| A��CO2��CO����ͨ������O2��ȼ |
| B��KNO3��Һ��AgNO3����������NaCl��Һ������ |
| C��NaCl��Һ��I2�����Ӿƾ�����Һ |
| D����ȥ�Ҵ����ܽ����ʳ�Σ����Բ�������ķ��� |
 ����MgCl2��Al2��SO4��3�����Һ�������в��ϼ���NaOH��Һ���õ��������������NaOH��Һ�������ͼ��ʾ��ԭ��Һ��Cl-��SO42-�����ʵ���֮��Ϊ ��������
����MgCl2��Al2��SO4��3�����Һ�������в��ϼ���NaOH��Һ���õ��������������NaOH��Һ�������ͼ��ʾ��ԭ��Һ��Cl-��SO42-�����ʵ���֮��Ϊ ��������| A��1��3 | B��2��3 |
| C��6��1 | D��3��1 |
�������£����������ͼ����Һ��Ϻ�pHһ������7���ǣ�������
| A��pH=3��HNO3��pH=11��KOH |
| B��pH=3�������pH=11�İ�ˮ |
| C��pH=3�����pH=11��NaOH |
| D��pH=3�Ĵ����pH=11��Ba��OH��2 |
ͨ����������һ������ʯ�ͻ�����չˮƽ��־���ǣ�������
| A����ϩ�IJ��� |
| B��ʯ�͵IJ��� |
| C��ԭú�IJ��� |
| D������IJ��� |
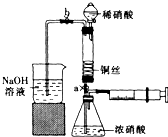 ijͬѧ��������װ��ʵ��ͭ��Ũ���ᡢϡ���ᷴӦ���������£�
ijͬѧ��������װ��ʵ��ͭ��Ũ���ᡢϡ���ᷴӦ���������£�