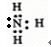��Ŀ����
X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ�
�ش��������⣺
��1��L��Ԫ�ط���Ϊ
��2��Z��X��Ԫ�ذ�ԭ����Ŀ��l��3��2��4���ɷ���A��B��A�ĵ���ʽΪ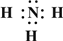
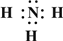 ��B�ĽṹʽΪ
��B�ĽṹʽΪ
 ��
��
��3������se��������������Ԫ�أ���Lͬһ���壬Seԭ�ӱ�Lԭ�Ӷ��������Ӳ㣬��Se��ԭ������Ϊ
a��+99.7mol?L-1 b��+29.7mol?L-1 c��-20.6mol?L-1 d��-241.8kJ?mol-1��
�ش��������⣺
��1��L��Ԫ�ط���Ϊ
O
O
��M��Ԫ�����ڱ��е�λ��Ϊ�������ڵڢ�A��
�������ڵڢ�A��
������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����Al��C��N��O��H
Al��C��N��O��H
����Ԫ�ط��ű�ʾ������2��Z��X��Ԫ�ذ�ԭ����Ŀ��l��3��2��4���ɷ���A��B��A�ĵ���ʽΪ
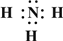
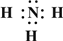


��3������se��������������Ԫ�أ���Lͬһ���壬Seԭ�ӱ�Lԭ�Ӷ��������Ӳ㣬��Se��ԭ������Ϊ
34
34
��������������Ӧ��ˮ���ﻯѧʽΪH2SeO4
H2SeO4
������2��5����Ԫ�ص��ʷֱ���H2��Ӧ����l mol��̬�⻯��ķ�Ӧ�����£���ʾ����1mol�����ⷴӦ�ȵ���b
b
������ĸ���ţ���a��+99.7mol?L-1 b��+29.7mol?L-1 c��-20.6mol?L-1 d��-241.8kJ?mol-1��
������X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ���XΪHԪ�أ�YΪCԪ�أ�ZΪNԪ�أ�LΪOԪ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ�MΪAlԪ�أ�
��1��LΪOԪ�أ�MΪAlԪ�أ��������ڱ��е������ڵڢ�A�壬����ͬ������ԭ����������ԭ�Ӱ뾶��С�����Ӳ�Խ��ԭ�Ӱ뾶Խ����ԭ�Ӱ뾶�ıȽϣ�
��2��ZΪNԪ�ء�XΪHԪ�أ�Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���AΪNH3��BΪN2H4��
��3������ͬ��Ԫ�أ�ԭ�������ĵݱ��Լ���Se��ԭ��������Se������ϼ�Ϊ+6��
�������ķ���ʽ��д����������Ӧ��ˮ���ﻯѧʽ��
ͬ�������϶��·ǽ����Լ�����������������Ӧ���ҳ̶ȼ�С����Ӧ�������Ƿ��ţ���
��1��LΪOԪ�أ�MΪAlԪ�أ��������ڱ��е������ڵڢ�A�壬����ͬ������ԭ����������ԭ�Ӱ뾶��С�����Ӳ�Խ��ԭ�Ӱ뾶Խ����ԭ�Ӱ뾶�ıȽϣ�
��2��ZΪNԪ�ء�XΪHԪ�أ�Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���AΪNH3��BΪN2H4��
��3������ͬ��Ԫ�أ�ԭ�������ĵݱ��Լ���Se��ԭ��������Se������ϼ�Ϊ+6��
�������ķ���ʽ��д����������Ӧ��ˮ���ﻯѧʽ��
ͬ�������϶��·ǽ����Լ�����������������Ӧ���ҳ̶ȼ�С����Ӧ�������Ƿ��ţ���
����⣺X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ���XΪHԪ�أ�YΪCԪ�أ�ZΪNԪ�أ�LΪOԪ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ�MΪAlԪ�أ�
��1��������������֪��LΪOԪ�أ�MΪAlԪ�أ��������ڱ��е������ڵڢ�A�壮ͬ������ԭ����������ԭ�Ӱ뾶��С�����Ӳ�Խ��ԭ�Ӱ뾶Խ����������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����Al��C��N��O��H��
�ʴ�Ϊ��O���������ڵڢ�A�壬Al��C��N��O��H��
��2��ZΪNԪ�ء�XΪHԪ�أ�Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���AΪNH3������ʽΪ��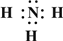 ��
��
BΪN2H4���ṹʽΪ ��
��
�ʴ�Ϊ��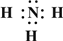 ��
�� ��
��
��3��Se��OԪ��ͬ��Ԫ�أ���Se��ԭ������Ϊ8+8+18=34��Se������ϼ�Ϊ+6������������Ӧ��ˮ���ﻯѧʽΪH2SeO4��ͬ�������϶��·ǽ����Լ�����������������Ӧ���ҳ̶ȼ�С����Ӧ�������Ƿ��ţ���������1mol�����⣨H2Se����Ӧ��Ӧ���ڵڶ�λ��ӦΪ+29.7kJ?mol-1����ѡb��
�ʴ�Ϊ��34��H2SeO4��b��
��1��������������֪��LΪOԪ�أ�MΪAlԪ�أ��������ڱ��е������ڵڢ�A�壮ͬ������ԭ����������ԭ�Ӱ뾶��С�����Ӳ�Խ��ԭ�Ӱ뾶Խ����������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����Al��C��N��O��H��
�ʴ�Ϊ��O���������ڵڢ�A�壬Al��C��N��O��H��
��2��ZΪNԪ�ء�XΪHԪ�أ�Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���AΪNH3������ʽΪ��
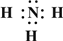 ��
��BΪN2H4���ṹʽΪ
 ��
���ʴ�Ϊ��
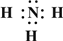 ��
�� ��
����3��Se��OԪ��ͬ��Ԫ�أ���Se��ԭ������Ϊ8+8+18=34��Se������ϼ�Ϊ+6������������Ӧ��ˮ���ﻯѧʽΪH2SeO4��ͬ�������϶��·ǽ����Լ�����������������Ӧ���ҳ̶ȼ�С����Ӧ�������Ƿ��ţ���������1mol�����⣨H2Se����Ӧ��Ӧ���ڵڶ�λ��ӦΪ+29.7kJ?mol-1����ѡb��
�ʴ�Ϊ��34��H2SeO4��b��
����������Ԫ���ƶϡ�����ʽ�ȳ��û�ѧ������д����Ӧ�ȱȽϵȣ�ע�⣨3���и��ݷ�Ӧ�ľ��ҳ̶ȳ̶��жϣ�

��ϰ��ϵ�д�
�����Ŀ