��Ŀ����
���ô�������Ϊ2���һ���1��3�������������л���A������ԭ�Ϻϳ����ô�������X��·�����£�

��֪��
(1)7.4 g�л���A��ȫȼ�տɲ���0.4 mol��CO2��0.5 mol��H2O��A����������������ܶ�Ϊ37���˴Ź���������ʾA����5��������ԭ�ӵ����շ壬�����֮��Ϊ3��3��2��2��1��
(2)

��ش��������⣺
(1)A�ķ���ʽ��________������������������________��
(2)�ڵĻ�ѧ��Ӧ������________��
(3)�ٵĻ�ѧ��Ӧ����ʽ��________��
(4)һ�������£���G����һ�ָ߷�����֬�Ļ�ѧ����ʽ��________��
(5)�����л���K�к�����Ԫ�ص�ʵ�������________________��
(6)X�ж���ͬ���칹�壬����ͬʱ��������������ͬ���칹����________�֣���д������һ�ֵĽṹ��ʽ________��
�ٱ�����������ȡ��������������Ϊ��ͬ���ţ�
��1 mol�л������2 mol��������ˮ��Һ��ȫ��Ӧ�õ������л����
�𰸣�

��ϰ��ϵ�д�
�����Ŀ

 ��X����±ԭ�ӣ�
��X����±ԭ�ӣ� +
+
 +H2O
+H2O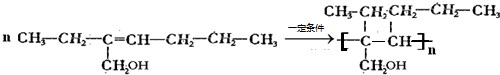




 ��X����±ԭ�ӣ�
��X����±ԭ�ӣ�