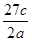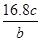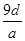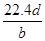��Ŀ����
ij����С�����Mg��CO2�ķ�Ӧԭ����̽��Mg��NO2�ķ�Ӧ����������С��ͨ��ʵ��ȷ��Mg����NO2��ȼ�գ����Թ������������ּ��裺
I��ͬ�����ΪMgO��
II������Ϊ______________��
III��������______________��
��ش��������⣺
������Ϣ��2NO2 + 2NaOH = NaNO3 + NaNO2 + H2O
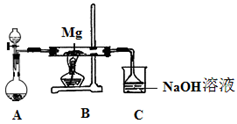
��1����ͼ���Ӻ�������װҩƷǰ��μ���װ�õ�������_______��

��2��װ��B��ʢװ�ĸ���������ǣ�����ţ�_______��
��Ũ���� ����ˮCaCl2 �ۼ�ʯ�� ������������
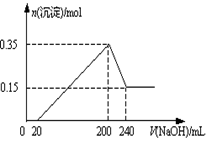
��3����ʼ����k����A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ�Ķ���______________��
��4��E���ռ��������������������ܶ���14����������_______��
��5��ʵ��õ�����������������ʵ��ǰMg��������1.5���������_______������C�з����Ļ�ѧ��Ӧ����ʽΪ______________
��6����ʵ���д�������ȱ�ݣ��Ľ���ʩ��___________
I��ͬ�����ΪMgO��
II������Ϊ______________��
III��������______________��
��ش��������⣺
������Ϣ��2NO2 + 2NaOH = NaNO3 + NaNO2 + H2O
��1����ͼ���Ӻ�������װҩƷǰ��μ���װ�õ�������_______��

��2��װ��B��ʢװ�ĸ���������ǣ�����ţ�_______��
��Ũ���� ����ˮCaCl2 �ۼ�ʯ�� ������������
��3����ʼ����k����A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ�Ķ���______________��
��4��E���ռ��������������������ܶ���14����������_______��
��5��ʵ��õ�����������������ʵ��ǰMg��������1.5���������_______������C�з����Ļ�ѧ��Ӧ����ʽΪ______________
��6����ʵ���д�������ȱ�ݣ��Ľ���ʩ��___________
�������ΪMg3N2
III���������ΪMgO��Mg3N2
��1���رշ�Һ©�������ͻ���K����������ĩ�˲���ˮ�У�����ƿ����C����Ӧ�ܣ��ȣ��������ܿ������ݣ�ֹͣ���ȣ��������н���һ��ˮ������ʾ���������á�
��2���ڢ�
��3���ž�װ���п�������ֹ��������ʵ�顣
��4��N2
��5������III 4Mg+2NO2
 4MgO+N2 3Mg+N2
4MgO+N2 3Mg+N2 Mg3N2
Mg3N2��6������K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
���������Mg��NO2��Ӧ������ԭ���غ㣬����������MgO��Mg3N2��MgO��Mg3N2
��1������װ��������ͨ���ü��ȷ����������������رգ�������ĩ������ˮ�У������巢��װ�ã����Ƿ�������ݣ�ֹͣ���Ⱥ��������Ƿ���һ��ˮ����
��2��NO2����Ũ���ᡢNaOH��Ӧ����˲�����Ũ���ᡢ��ʯ�Ҹ��
��3��Ϊ��ֹMg������е�N2��O2��Ӧ��ʵ��������ţ����ž�װ���п�����
��4���ռ������������������ܶ���14�����������Է���������2��14=28������ΪN2��
��5��������ֻ��MgO������������Ƿ�Ӧǰ��
 ��1.7����������ֻ��Mg3N2�������������Ƿ�Ӧǰ��
��1.7����������ֻ��Mg3N2�������������Ƿ�Ӧǰ�� ��1.4����ʵ��õ��Ĺ��������Ƿ�Ӧǰ��1.5������õ�����MgO��Mg3N2�Ļ����ʼ���III������
��1.4����ʵ��õ��Ĺ��������Ƿ�Ӧǰ��1.5������õ�����MgO��Mg3N2�Ļ����ʼ���III����������Mg��CO2�ķ�Ӧ����֪Mg��NO2��Ӧ����MgO��N2������N2������Mg��Ӧ�õ�Mg3N2��
��6���Ƚ����Ե�ȱ���ǣ��ž�װ�ÿ���ʱ��������NO2�ڻ���k��ֱ����������У�����Ⱦ����������轫����k���ĵ���ͨ��NaOH��Һ�С�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 2MgSO3+S����ͬѧ���Ʋ��ǣ�3Mg+SO2
2MgSO3+S����ͬѧ���Ʋ��ǣ�3Mg+SO2 3Ba��+BaO��Al2O3
3Ba��+BaO��Al2O3