ΧβΡΩΡΎ»ί
Θ®10Ζ÷Θ©
Ρ≥Ά§―ß‘Ύ”ΟœΓΝρΥα”κ–ΩΝΘΘ®ΜΤΕΙΝΘ¥σ–ΓΘ©÷Τ»Γ«βΤχΒΡ Β―ι÷–Θ§ΖΔœ÷Φ”»κ…ΌΝΩΝρΥαΆ≠»ή“ΚΩ…Φ”Ωλ«βΤχΒΡ…ζ≥…ΥΌ¬ ΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©…œ ω Β―ι÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ”– ΘΜ
Θ®2Θ©ΝρΥαΆ≠»ή“ΚΩ…“‘Φ”Ωλ«βΤχ…ζ≥…ΥΌ¬ ΒΡ‘≠“ρ « ΘΜ
Θ®3Θ©“ΣΦ”Ωλ…œ ω Β―ι÷–ΤχΧε≤ζ…ζΒΡΥΌ¬ Θ§ΜΙΩ…≤…»ΓΒΡ¥κ ©”–
ΓΔ ΓΔ ΓΘ
Θ®4Θ©ΈΣΝΥΫχ“Μ≤Ϋ―–ΨΩΝρΥαΆ≠ΒΡΝΩΕ‘«βΤχ…ζ≥…ΥΌ¬ ΒΡ”ΑœλΘ§ΗΟΆ§―ß…ηΦΤΝΥ»γœ¬“ΜœΒΝ– Β―ιΓΘΫΪ±μ÷–ΥυΗχΒΡΜλΚœ»ή“ΚΖ÷±πΦ”»κΒΫ6Ηω Δ”–ΙΐΝΩZnΝΘΒΡΖ¥”ΠΤΩ÷–Θ§ ’Φ·≤ζ…ζΒΡΤχΧεΘ§Φ«¬ΦΜώΒΟœύΆ§ΧεΜΐΒΡΤχΧεΥυ–ηΒΡ ±ΦδΓΘ
«κΆξ≥…¥Υ Β―ι…ηΦΤΘ§Τδ÷–ΘΚV1= Θ§V6= Θ§V9= ΘΜ
|
Β ―ι ΜλΚœ»ή“Κ
|
A
|
B
|
C
|
D
|
E
|
F
|
|
|
4mol/LH2SO4/mL
|
40 |
V1 |
V2 |
V3 |
V4 |
V5 | |
|
CuSO4/mL |
0 |
1 |
5 |
10 |
V6 |
40 | |
|
H2O/mL |
V7 |
V8 |
V9 |
V10 |
15 |
0 |
ΗΟΆ§―ßΉνΚσΒΟ≥ωΒΡΫα¬έΈΣΘΚΒ±Φ”»κ…ΌΝΩ![]() »ή“Κ ±Θ§…ζ≥…«βΤχΒΡΥΌ¬ Μα¥σ¥σΧαΗΏΓΘΒΪΒ±Φ”»κΒΡ
»ή“Κ ±Θ§…ζ≥…«βΤχΒΡΥΌ¬ Μα¥σ¥σΧαΗΏΓΘΒΪΒ±Φ”»κΒΡ![]() »ή“Κ≥§Ιΐ“ΜΕ®ΝΩ ±Θ§…ζ≥…«βΤχΒΡΥΌ¬ Ζ¥ΕχΜαœ¬ΫΒΓΘ
»ή“Κ≥§Ιΐ“ΜΕ®ΝΩ ±Θ§…ζ≥…«βΤχΒΡΥΌ¬ Ζ¥ΕχΜαœ¬ΫΒΓΘ
Θ®1Θ©Zn+H2SO4=ZnSO4+H2Γϋ Zn+CuSO4=ZnSO4+Cu
Θ®2Θ©Zn”κCuSO4»ή“ΚΖ¥”Π÷ΟΜΜ≥ωCuΘ§ ZnΓΔCuΓΔH2SO4ΙΙ≥…‘≠Βγ≥ΊΘ§Φ”ΩλΖ¥”ΠΥΌ¬ ΓΘ
Θ®3Θ©Φ”»»ΘΜ Β±‘ω¥σΝρΥαΒΡ≈®Ε»ΘΜΫΪ–ΩΝΘΗΡ≥…–ΩΖέΘ§‘ω¥σΫ”¥ΞΟφΜΐΒ» Θ®ΚœάμΦ¥Ω…Θ©
Θ®4Θ©V1 =40Θ§V6 = 25Θ§V9 =35
ΫβΈω:

 ΥΪΜυΆ§≤ΫΒΦΚΫ―ΒΝΖœΒΝ–¥πΑΗ
ΥΪΜυΆ§≤ΫΒΦΚΫ―ΒΝΖœΒΝ–¥πΑΗ ΜΤΗ‘–ΓΉ¥‘ΣΆ§≤ΫΦΤΥψΧλΧλΝΖœΒΝ–¥πΑΗ
ΜΤΗ‘–ΓΉ¥‘ΣΆ§≤ΫΦΤΥψΧλΧλΝΖœΒΝ–¥πΑΗ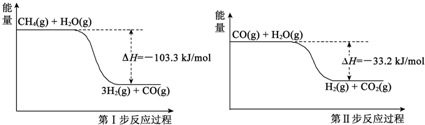 «βΤχ «“Μ÷÷«εΫύΡή‘¥Θ§«βΤχΒΡ÷Τ»Γ”κ¥Δ¥φ ««βΡή‘¥άϊ”ΟΝλ”ρΒΡ―–ΨΩ»»ΒψΘ°
«βΤχ «“Μ÷÷«εΫύΡή‘¥Θ§«βΤχΒΡ÷Τ»Γ”κ¥Δ¥φ ««βΡή‘¥άϊ”ΟΝλ”ρΒΡ―–ΨΩ»»ΒψΘ° ΓΔ
ΓΔ ΓΔ
ΓΔ ΓΔ
ΓΔ Β»4÷–»ή“ΚΘ§Ω…”κ Β―ι÷–
Β»4÷–»ή“ΚΘ§Ω…”κ Β―ι÷– »ή“ΚΤπœύΥΤΉς”ΟΒΡ « ΘΜ
»ή“ΚΤπœύΥΤΉς”ΟΒΡ « ΘΜ
 ΓΔ
ΓΔ ΓΔ
ΓΔ ΓΔ
ΓΔ Β»4÷–»ή“ΚΘ§Ω…”κ Β―ι÷–
Β»4÷–»ή“ΚΘ§Ω…”κ Β―ι÷– »ή“ΚΤπœύΥΤΉς”ΟΒΡ «
ΘΜ
»ή“ΚΤπœύΥΤΉς”ΟΒΡ «
ΘΜ