��Ŀ����
Na2SO3��7H2O��ʳƷ��ҵ�г��õ�Ư�������������ͷ�������Na2SO3��30��ʱ���ܽ��Ϊ
35��5g/(100gH2O)��
��1������30��ʱNa2SO3������Һ��Na2SO3������������������2λС����
��2������30��ʱ271g Na2SO3������Һ��ˮ��������
��3����30���Na2SO3������Һ271g��ȴ��10�棬����Na2SO3��7H2O����79��5g������10��ʱNa2SO3��ˮ�е��ܽ�ȡ�
35��5g/(100gH2O)��
��1������30��ʱNa2SO3������Һ��Na2SO3������������������2λС����
��2������30��ʱ271g Na2SO3������Һ��ˮ��������
��3����30���Na2SO3������Һ271g��ȴ��10�棬����Na2SO3��7H2O����79��5g������10��ʱNa2SO3��ˮ�е��ܽ�ȡ�
��1��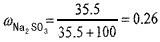
��2��200g
��3��19.5g/(100gH2O)
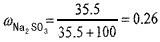
��2��200g
��3��19.5g/(100gH2O)

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��������2λС����
��������2λС���� ��������2λС����
��������2λС����