��Ŀ����
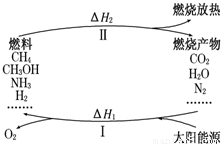
����������Ŀǰ�������ٵ�һ���ش���⣮ΪӦ��ȼ��ʹ����ɵĻ�����Ⱦ����ѧ�ҹ���������̫���ܴٽ�ȼ�ϵ�ѭ��ʹ�ã��乹�������ͼ��ʾ��
������Ҫ�ķ�ӦΪ��
��2CO2
2CO+O2
��2H2O
2H2+O2
��2N2+6H2O
4NH3+3O2
��2CO2+4H2O
2CH3OH+3O2
��______+H2O
CH4+______��
������գ�
��1�����㽫��Ӧ�ݲ���������______+H2O
CH4+______
��2�����̢��еġ�H______0�����������������=������
��3�����жԹ��̢�͢����������ȷ����______��
a��̫��������ת��Ϊ��ѧ�������ڻ�ѧ������
b��̫����������Ҫת��Ϊ����
c����������һ��ѭ����ų�O2
d����������һ��ѭ�������������
��4��Ҫʵ������ѭ������ǰ��Ҫ����Ĺؼ������ǹ���______��������˹��̵���ƿɲο���Ȼ����______���ã�
��5����CH4����ԭNOx�������������������Ⱦ�����磺
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ?mol-1
���ñ�״����4.48L CH4��ԭNO2��N2������������ת�Ƶ��ӵ����ʵ���Ϊ______����д������NO2ת��ΪN2���Ȼ�ѧ����ʽ��______��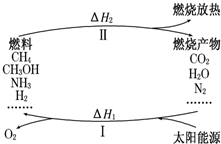
������Ҫ�ķ�ӦΪ��
��2CO2
| ||
��2H2O
| ||
��2N2+6H2O
| ||
��2CO2+4H2O
| ���� |
��______+H2O
| ���� |
������գ�
��1�����㽫��Ӧ�ݲ���������______+H2O
| ���� |
��2�����̢��еġ�H______0�����������������=������
��3�����жԹ��̢�͢����������ȷ����______��
a��̫��������ת��Ϊ��ѧ�������ڻ�ѧ������
b��̫����������Ҫת��Ϊ����
c����������һ��ѭ����ų�O2
d����������һ��ѭ�������������
��4��Ҫʵ������ѭ������ǰ��Ҫ����Ĺؼ������ǹ���______��������˹��̵���ƿɲο���Ȼ����______���ã�
��5����CH4����ԭNOx�������������������Ⱦ�����磺
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ?mol-1
���ñ�״����4.48L CH4��ԭNO2��N2������������ת�Ƶ��ӵ����ʵ���Ϊ______����д������NO2ת��ΪN2���Ȼ�ѧ����ʽ��______��

��1��������̼��ˮ��Ӧ���ɼ�����������ʴ�Ϊ��CO2��2O2��
��2�����̢���ȼ�շ�Ӧ�������ȣ��ʡ�H��0���ʴ�Ϊ������
��3��a���ۺ������̣��佫��ѧ��ת��Ϊ����ʹ�ã���a����
b���ۺ������̣��佫��ѧ��ת��Ϊ����ʹ�ã���b��ȷ��
C��һ��ѭ����û�����������ɣ���C����
d��һ��ѭ����û�����������ɣ���d��ȷ��
��ѡbd��
��4����ǰ��Ҫ����Ĺؼ��ǹ��̢˹��̵���ƿɲο���Ȼ���й�����ã��ʴ�Ϊ����ϣ�
��5����֪��CH4��g��+4N02��g��=4NO��g��+C02��g��+2H20��g����H=-574kJ?mol-1��
��CH4��g��+4N0��g��=2N2��g��+C02��g��+2H20��g����H=-1160kJ?mol-1��
��ӦCH4��g��+2N02��g��=N2��g��+C02��g��+2H20��g������
����+�ڣ���
���ݸ�˹���ɣ����Է�ӦCH4��g��+2N02��g��=N2��g��+C02��g��+2H20��g����H=
[��-574kJ?mol-1��+��-1160kJ?mol-1��]=-867KJ/mol����ӦCH4��g��+2N02��g��=N2��g��+C02��g��+2H20��g���У�1mol����μӷ�Ӧת�Ƶ�����Ϊ8mol�����Ա�״����4.48L��0.2molCH4��ԭNO2��N2����������ת�Ƶĵ���Ϊ1.6mol��
�ʴ�Ϊ��1.6 mol��CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H=-867kJ?mol-1��
��2�����̢���ȼ�շ�Ӧ�������ȣ��ʡ�H��0���ʴ�Ϊ������
��3��a���ۺ������̣��佫��ѧ��ת��Ϊ����ʹ�ã���a����
b���ۺ������̣��佫��ѧ��ת��Ϊ����ʹ�ã���b��ȷ��
C��һ��ѭ����û�����������ɣ���C����
d��һ��ѭ����û�����������ɣ���d��ȷ��
��ѡbd��
��4����ǰ��Ҫ����Ĺؼ��ǹ��̢˹��̵���ƿɲο���Ȼ���й�����ã��ʴ�Ϊ����ϣ�
��5����֪��CH4��g��+4N02��g��=4NO��g��+C02��g��+2H20��g����H=-574kJ?mol-1��
��CH4��g��+4N0��g��=2N2��g��+C02��g��+2H20��g����H=-1160kJ?mol-1��
��ӦCH4��g��+2N02��g��=N2��g��+C02��g��+2H20��g������
| 1 |
| 2 |
���ݸ�˹���ɣ����Է�ӦCH4��g��+2N02��g��=N2��g��+C02��g��+2H20��g����H=
| 1 |
| 2 |
�ʴ�Ϊ��1.6 mol��CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H=-867kJ?mol-1��

��ϰ��ϵ�д�
�����Ŀ
 ����������Ŀǰ�������ٵ�һ���ش���⣮ΪӦ��ȼ��ʹ����ɵĻ�����Ⱦ����ѧ�ҹ���������̫���ܴٽ�ȼ�ϵ�ѭ��ʹ�ã��乹�������ͼ��ʾ��
����������Ŀǰ�������ٵ�һ���ش���⣮ΪӦ��ȼ��ʹ����ɵĻ�����Ⱦ����ѧ�ҹ���������̫���ܴٽ�ȼ�ϵ�ѭ��ʹ�ã��乹�������ͼ��ʾ�� 2CO+O2
2CO+O2 2H2+O2
2H2+O2 4NH3+3O2
4NH3+3O2 2CH3OH+3O2
2CH3OH+3O2 CH4+ ��
CH4+ �� CH4+
CH4+