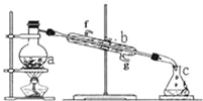
��Ŀ����
����Ŀ���ڱ�״���£���������������Ȫʵ�飬������������д��ƿ����Һ�����ʵ���Ũ�ȣ�������ƿ�����ʲ�����ɢ��������
��1����HCl��������Ȫʵ�飬��Ȫ������ˮ������ƿ������Һ�����ʵ���Ũ��Ϊ_______��
��2����NH3����Ȫʵ��ʱ����Ȫ������ˮ������ƿ������Һ�����ʵ���Ũ��Ϊ____________��
��3����NO2����Ȫʵ��ʱ����Ȫ������ˮ������ƿ��![]() ��������Һ�����ʵ���Ũ��Ϊ____________��
��������Һ�����ʵ���Ũ��Ϊ____________��
��4����NO2��O2��4��1����Ȼ�ϣ���Ȫ������ˮ������ƿ������Һ�����ʵ���Ũ��Ϊ________��
���𰸡�0.045 mol��L��1 0.045 mol��L��1 0.045 mol��L��1 0.036 mol��L��1
��������
��1������HCl���弫������ˮ������������Һ�������ԭHCl����������ȣ����൱��VL��Һ���ܽ���VL HCl���壬��HCl��������ʵ���Ϊ![]() mol������ƿ���ΪVL������Һ�����ʵ���Ũ��Ϊ
mol������ƿ���ΪVL������Һ�����ʵ���Ũ��Ϊ![]() mol��VL��
mol��VL��![]() mol��L��1��0.045 mol��L��1��
mol��L��1��0.045 mol��L��1��
�ʴ�Ϊ��0.045 mol��L��1
��2������NH3Ҳ��������ˮ������������Һ�������ԭNH3���������ȵġ���������Һ�����ʵ���Ũ��ҲΪ![]() mol��L��1��ԼΪ0.045 mol��L��1��
mol��L��1��ԼΪ0.045 mol��L��1��
�ʴ�Ϊ��0.045 mol��L��1��
��3���ɷ�Ӧ����ʽ3NO2��H2O��2HNO3��NO֪��NO2������![]() ��������HNO3����Ȫʵ�������������Һ�������ΪԭNO2���������
��������HNO3����Ȫʵ�������������Һ�������ΪԭNO2���������![]() ���ʸ���Һ�����ʵ���Ũ��Ϊ
���ʸ���Һ�����ʵ���Ũ��Ϊ![]() mol��L��1��ԼΪ0.045 mol��L��1��
mol��L��1��ԼΪ0.045 mol��L��1��
�ʴ�Ϊ��0.045 mol��L��1��
��4���ɷ�Ӧ����ʽ4NO2��O2��2H2O��4HNO3֪��NO2��HNO3�����ʵ�����ȣ�NO2�����ռ��������������![]() �����൱��1 L��Һ�����е�HNO3Ϊ
�����൱��1 L��Һ�����е�HNO3Ϊ![]() mol��ԼΪ0.036 mol����c��HNO3����0.036 mol��L��1��
mol��ԼΪ0.036 mol����c��HNO3����0.036 mol��L��1��
�ʴ�Ϊ��0.036 mol��L��1��

 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�����Ŀ����SO2�ֱ�ͨ��������������Ũ�Ⱦ�Ϊ0.1 mol��L��1�� BaCl2��Һ��Ba(NO3)2��Һ�У�̽����ϵ�����������ã�ʵ���¼���£�
ʵ���¼ | |
pH�仯 |
|
��Һ���Ƿ�������� | BaCl2��Һ(����)���ް�ɫ������BaCl2��Һ(����)���а�ɫ���� Ba(NO3)2��Һ(����)���а�ɫ������Ba(NO3)2��Һ(����)���а�ɫ���� |
����˵������ȷ����
A.����a ��ʾ��ҺpH���͵�ԭ��SO2 + H2O![]() H2SO3
H2SO3![]() H+ + HSO3-
H+ + HSO3-
B.����c ��ʾ��Һ�з�����Ӧ��2Ba2+ + O2 + 2SO2 + 2H2O =2BaSO4��+ 4H+
C.������a��b��c�Աȣ���֪����d����ʾ�Ĺ�����NO3-������SO2����Ҫ��
D.���ݸ�ʵ��Ԥ��0.2 mol��L��1��KNO3��Һ��������Ҳ��������SO2
����Ŀ��������Ȼ���������γɵĹ����У�ָ��Ԫ�ؼ�û����������û������ԭ����
|
|
|
|
A���ܶ������� | B�����硪���� | C����ɽ�緢������ | D��������á���̼ |
A.AB.BC.CD.D
����Ŀ��800��ʱ��������ͬ�ĺ����ܱ������з�����ӦCO(g) + H2O(g)![]() CO2(g) + H2(g) K��һ��ʱ��ֱ�ﵽ��ѧƽ��״̬��
CO2(g) + H2(g) K��һ��ʱ��ֱ�ﵽ��ѧƽ��״̬��
������� | ��ʼŨ��/(mol��L1) | |||
c(CO) | c(H2O) | c(CO2) | c(H2) | |
�� | 0.01 | 0.01 | 0 | 0 |
�� | 0 | 0 | 0.01 | 0.01 |
III | 0.008 | 0.008 | 0.002 | 0.002 |
����˵������ȷ����
A.�� �д�ƽ��ʱ��c(H2)��0.005 mol��L1
B.III�д�ƽ��ʱ��CO�������������25%
C.III�дﵽƽ��״̬�����ʱ��� �� �еĶ�
D.��III����ʼŨ�Ⱦ�����һ����ƽ��ʱc(H2)������һ��