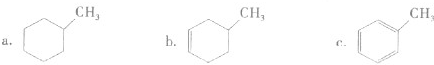
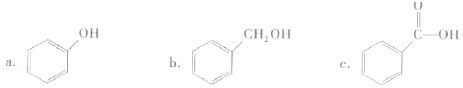
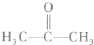
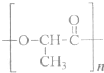��Ŀ����
�л���A���������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������AΪ��ɫճ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
| ʵ �� �� �� | �� �� �� ʵ �� �� �� |
| ��1����ȡA 9.0g������ʹ�������������ܶ�����ͬ������H2��45���� | ��ͨ��������գ� ��1��A��Ħ������Ϊ��___________�� |
| ��2������9.0gA��������O2���ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ң��������߷ֱ�����5.4g��13.2g�� | ��2��A�ķ���ʽΪ��_____________�� |
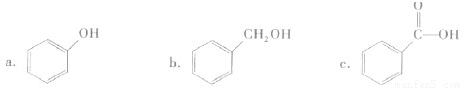
| ��3����ȡA 9.0g����������NaHCO3��ĩ��Ӧ������2.24LCO2����״�������������������Ʒ�Ӧ������2.24LH2����״������ | ��3���ýṹ��ʽ��ʾA�к��еĹ����ţ� ___________________________�� |
| ��4��A�ĺ˴Ź�����������ͼ��
| ��4��A�к���___________����ԭ�ӡ� |
| ��5������������A�Ľṹ��ʽ__________________�� | |
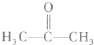
��1��90 g/mol ��2��C3H6O3��3����COOH����OH ��4��4��5��CH3CH��OH��COOH
����:
������

��ϰ��ϵ�д�
�����Ŀ