��Ŀ����
���з�Ӧ�У�һ�������Է����е���
A.2KClO3(s)= 2KCl(s)+ 3O2(g) ��H = ��78.03 kJ��mol��1����S = 494.4 J��mol��1��K��1
B.CO(g)=
C(s,ʯī)+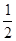 O2(g)
��H = 110.5
kJ��mol��1����S = ��89.4 J��mol��1��K��1
O2(g)
��H = 110.5
kJ��mol��1����S = ��89.4 J��mol��1��K��1
C.4Fe(OH)2(s)+2H2O(l)+O2(g)= 4Fe(OH)3(s)
��H = ��444.3 kJ��mol��1����S = ��280.1 J��mol��1��K��1
D.NH4HCO3(s) + CH3COOH(aq) = CH3COONH4 (aq) + CO2(g) +H2O(l)
��H = 37.30 kJ��mol��1����S = 184.0 J��mol��1��K��1
���𰸡�
B
����������

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ