��Ŀ����
��������Ԫ�أ�����A��B��C��DΪ����������Ԫ�أ�E��FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
(1)A�Ļ�̬ԭ���������______�ֲ�ͬ�˶�״̬�ĵ��ӡ�
(2)E2���Ļ�̬��������Ų�ʽΪ____________________��
(3)A��B��C����Ԫ�ص�����⻯����۵��ɵ͵��ߵ�˳����________��A��B��C����Ԫ����ɵķ�������AC2��Ϊ�ȵ�����ķ���Ϊ________________(��Ԫ�ط��ű�ʾ)��
(4)BD3����ԭ�ӵ��ӻ���ʽΪ________������ӿռ乹��Ϊ____________��
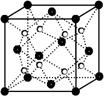
(5)�þ����X�������䷨��F�IJⶨ�õ����½����F�ľ���Ϊ�����������ܶѻ�(����ͼ)����֪�þ�����ܶ�Ϊ�� g��cm��3�������и�ԭ�ӵ���λ��Ϊ______________��F��ԭ�Ӱ뾶��________ cm(�����ӵ�����ΪNA)��

| Aԭ�Ӻ�����ӷ�ռ3����ͬ�ܼ�����ÿ���ܼ����Ų��ĵ�������ͬ |
| BԪ��ԭ�ӵĺ���p����������s����������1 |
| Cԭ��p����ϳɶԵ���������δ�ɶԵ�����������Aͬ���� |
| DԪ�ص����������������IJ�Ϊ4���Ҳ���AԪ����ͬһ���� |
| Eλ�����ڱ��е����� |
| FԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��� |
(1)A�Ļ�̬ԭ���������______�ֲ�ͬ�˶�״̬�ĵ��ӡ�
(2)E2���Ļ�̬��������Ų�ʽΪ____________________��
(3)A��B��C����Ԫ�ص�����⻯����۵��ɵ͵��ߵ�˳����________��A��B��C����Ԫ����ɵķ�������AC2��Ϊ�ȵ�����ķ���Ϊ________________(��Ԫ�ط��ű�ʾ)��
(4)BD3����ԭ�ӵ��ӻ���ʽΪ________������ӿռ乹��Ϊ____________��
(5)�þ����X�������䷨��F�IJⶨ�õ����½����F�ľ���Ϊ�����������ܶѻ�(����ͼ)����֪�þ�����ܶ�Ϊ�� g��cm��3�������и�ԭ�ӵ���λ��Ϊ______________��F��ԭ�Ӱ뾶��________ cm(�����ӵ�����ΪNA)��

(1)4��
(2)1s22s22p63s23p63d5��[Ar]3d5
(3)CH4��NH3��H2O��N2O
(4)sp3��������
(5)12��
(2)1s22s22p63s23p63d5��[Ar]3d5
(3)CH4��NH3��H2O��N2O
(4)sp3��������
(5)12��

A�ĵ����Ų�ʽΪ1s22s22p2��ΪCԪ�أ�B�ĵ����Ų�ʽΪ1s22s22p3��ΪNԪ�أ�C�ĵ����Ų�ʽΪ1s22s22p4��ΪOԪ�أ�DΪClԪ�أ�EΪMnԪ�أ�FΪCuԪ�ء�
(1)�м������Ӿ��м����˶�״̬������CԪ�ص������Ӧ��4�ֲ�ͬ�˶�״̬�ĵ��ӡ�
(3)����CH4��NH3��H2O������H2O��NH3�����ڷ��Ӽ�����������۵�H2O��NH3��CH4����C��N��O��ɵķ�������CO2��Ϊ�ȵ�����ķ���ΪN2O��
(4)NCl3��Nԭ�Ӳ�ȡsp3�ӻ�������ӿռ乹��Ϊ�����Ρ�
(5)ͭԭ�Ӱ뾶Ϊ��Խ��ߵ� �����ⳤΪa��
�����ⳤΪa��
��a3���ѡ�NA��4��64

(1)�м������Ӿ��м����˶�״̬������CԪ�ص������Ӧ��4�ֲ�ͬ�˶�״̬�ĵ��ӡ�
(3)����CH4��NH3��H2O������H2O��NH3�����ڷ��Ӽ�����������۵�H2O��NH3��CH4����C��N��O��ɵķ�������CO2��Ϊ�ȵ�����ķ���ΪN2O��
(4)NCl3��Nԭ�Ӳ�ȡsp3�ӻ�������ӿռ乹��Ϊ�����Ρ�
(5)ͭԭ�Ӱ뾶Ϊ��Խ��ߵ�
 �����ⳤΪa��
�����ⳤΪa����a3���ѡ�NA��4��64


��ϰ��ϵ�д�
�����Ŀ
 ͼa
ͼa






 ����1����
����1���� ����C6H6�ǷǼ��Է���
����C6H6�ǷǼ��Է���