��Ŀ����
����Ŀ��ij�о���ѧϰС������˽��� MnO2(���н϶�� MnO �� MnCO3)��Ʒת��Ϊ�� MnO2 ʵ�飬 ������������

(1)�ڢٲ���ϡ H2SO4 ʱ���� MnO2 ��Ʒ�е�______(д��ѧʽ)ת��Ϊ���������ʡ�
(2)�ڢڲ���Ӧ�����ӷ���ʽ�� + ClO3+ = MnO2��+ Cl2��+ ����ƽ___________________________________________________
(3)�ڢ۲������������������������̨(����Ȧ)���ƾ�����_____��_____����֪�����õ��Ĺ������� NaClO3 �� NaOH����һ�����к���_____(д��������)��
(4)���� MnO2 ��Ʒ������Ϊ 12.69g���ڢٲ���Ӧ���������˵õ� 8.7gMnO2�����ռ��� 0.224 L CO2(�� ״����)�����ڵڢڲ���Ӧ��������Ҫ______molNaClO3��
���𰸡�(1)MnO��MnCO3
(2)5 Mn2��2 4 H2O 5 1 8 H��
(3)�ƾ��ơ�������������NaCl
(4)0.02
��������
������MnO2���ᴿΪ����������������ԭ��Ӧ����ʽ����ƽ�����ʵķ������ᴿ�����ʵ������йؼ����֪ʶ��(1)������������ͼ֪��Ʒ�е�MnO��MnCO3����ϡ���ᷴӦת��ΪMnSO4��������Һ��(2)�û��ϼ�����������д������ƽ��������Ӧ�����ӷ���ʽ��5Mn2����2ClO3����4H2O=5MnO2����Cl2����8H����(3)Cl2ͨ����NaOH��Һ�У����ɵ�NaClO3��Ȼ�����������ô0�۵���Ԫ�ػ��ϼ۽������ɵĻ�ԭ�����Ȼ��NaCl��(4)n(MnCO3)��n(CO2)��![]() ��0.01 mol��n(MnO)��
��0.01 mol��n(MnO)��![]() ��0.04 mol����MnO��MnCO3����ϡ�������ɵ�Mn2�������ʵ���n(Mn2��)��n(MnO)��n(MnCO3)��0.05 mol���ɵ�������Ӧ��ѧ����ʽ֪����Mn2��������NaClO3��
��0.04 mol����MnO��MnCO3����ϡ�������ɵ�Mn2�������ʵ���n(Mn2��)��n(MnO)��n(MnCO3)��0.05 mol���ɵ�������Ӧ��ѧ����ʽ֪����Mn2��������NaClO3��![]() ��0.05 mol��0.02 mol��
��0.05 mol��0.02 mol��

 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�����Ŀ�����в���ǰ36��Ԫ�ص����ʻ�ԭ�ӽṹ���±�
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
R | ��̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2������ |
S | ��������ˮ���ҷ�Ӧ��������Һ�������� |
T | ��̬ԭ��3d�������1������ |
X | �� |
��1��RԪ�صĵ�һ������Ҫ������ͬ�������ڵ�Ԫ�أ�ԭ����________________________________________________________��
��2��SԪ�صĻ��ϼ��Ƿ������ۣ�__________��ԭ����__________________________________�����������Ų�ʽΪ________________________��
��3��TԪ�ص�ԭ��N�ܲ��ϵ�����Ϊ__________����ԭ�ӽṹʾ��ͼΪ__________��
��4��X�ĺ�������Ų�ͼΥ����__________����X���ʡ�������μ����������εȿ����������ȼ��ʱ�����������ɫ�Ĺ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ��____________________________________________________________________��
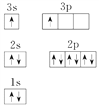
���𰸡� ��ԭ��2p���������������ͣ��ȶ� �� F�ĵ縺�����ֻ�ܵõ��� 2s22p5 2  �������ԭ�� ���Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
�������ԭ�� ���Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
�����������������RԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ�R��NԪ�أ�SԪ�صĵ�������ˮ���ҷ�Ӧ��������Һ�������ԣ�S��FԪ�أ�TԪ�صĻ�̬ԭ��3d�������1�����ӣ�T��21��Ԫ��Sc�� XԪ�ص�ԭ�Ӻ�����12�����ӣ�X��MgԪ�ء�
�������������Ϸ�������1��R��NԪ������ԭ��2p���������������ͣ��ȶ�,���Ե�һ������Ҫ������ͬ�������ڵ�OԪ����
��2��Ԫ��F�ĵ縺�����ֻ�ܵõ���������FԪ��û�����ۣ�FԪ�ص����������Ų�ʽΪ2s22p5��
��3��Scԭ�ӵĺ�������Ų�ʽ��1s22s22p63s23p63d14s2������N�ܲ��ϵ�����Ϊ2����ԭ�ӽṹʾ��ͼΪ ����4�������������ԭ����Mgԭ�������2������Ӧ�Ų���3s����������Ժ�������Ų�ͼΥ�����������ԭ�������Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����������ȼ�����ʱ�����������ɫ�Ĺ���
����4�������������ԭ����Mgԭ�������2������Ӧ�Ų���3s����������Ժ�������Ų�ͼΥ�����������ԭ�������Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����������ȼ�����ʱ�����������ɫ�Ĺ���
�����͡�������
��������
20
����Ŀ�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
T | ��������ˮ���ҷ�Ӧ��������Һ�������� |
X | L��p��������s��������2�� |
Y | ��������Ԫ�صļ������а뾶��С |
Z | L��������δ�ɶԵ��� |
��1��д��Ԫ��X�����ӽṹʾ��ͼ__________��
��2��д��YԪ������������ˮ����ֱ���HCl��NaOH��Һ��Ӧ�����ӷ���ʽ_______________________��_________________________��
��3��д��Z��Y�ĵ����Ų�ʽ______________��________________��
��4��Ԫ��T����Ԫ����ȣ��ǽ����Խ�ǿ����__________(��Ԫ�ط��ű�ʾ)�����б�������֤����һ��ʵ����__________��
A����̬�⻯��Ļӷ��Ժ��ȶ���
B�����ʷ����еļ���
C����Ԫ�صĵ縺��
D�������������
E���⻯����X��H���ļ���(X����T��Cl����Ԫ��)
F������������Ȼ���еĴ�����ʽ
��5��̽Ѱ���ʵ����ʲ�������ѧϰ����Ҫ����֮һ��T��X��Y��Z����Ԫ�صĵ����л�ѧ�������Բ�ͬ���������ֵ��ʵ���__________(��Ԫ�ط���)��������________________________________________________��
����Ŀ���� 100mL BaCl2��AlCl3 �� FeCl3 �Ļ����Һ A ����μ��� Na2SO4 �� NaOH �Ļ����Һ B�������ij��� �����ʵ���(n)�ͼ�����Һ B �����(V)��ϵ��ͼ��

(1)������ B ��Һ 110mL ʱ����Һ�еij���Ϊ_____(�ѧʽ����ͬ)����Һ�е�������____________________��
(2)�� 90mL �� 100 mL ֮����� 10mL B ��Һʱ���������ӷ�Ӧ����ʽ______��
(3)�� A��B ��Һ�и����ʵ����ʵ���Ũ�������±�
���� | Na2SO4 | NaOH | BaCl2 | AlCl3 | FeCl3 |
c(mol/L) | ___________ | ___________ | ___________ | ___________ | ___________ |