��Ŀ����
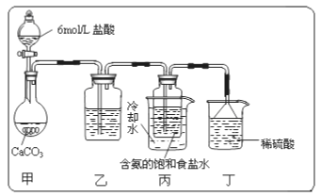
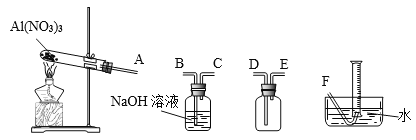
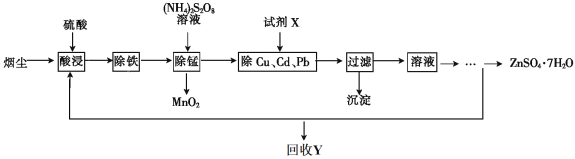
����Ŀ������ѧ����ѡ��2��ѧ�뼼������������ͭ�̳������������ս�ȡ���ȿɱ��������ֿɵõ���;�㷺�ĸߴ�����п����֪�̳�����Ҫ��ZnO������������FeO��Fe2O3��CuO��MnO��PbO��CdO���Ʊ������������£�
��֪����ؽ������������������������pH��������ȫ��pH���±���
�������� | ��ʼ������pH | ������ȫ��pH |
Fe3+ | 1��1 | 3��2 |
Zn2+ | 5��5 | 8��0 |
Fe2+ | 5��8 | 8��8 |
��1�����յIJ�ƷY��_____________________��
��2����������ʱ���ȼ���_________________������������ԭ�������ٽ���Һ��pH������__________��
��3�������̡�ʱ��Һ�з�����Ӧ�Ļ�ѧ����ʽΪ____________________��
��4����ȥCu��Cd��Pb��ͬʱ���Ի���һЩ�ؽ������Լ�XΪ__________________��
��5��ZnSO4��Һ����__________��__________�����˼�����õ�ZnSO4��7H2O��
��6��Ϊ�˲ⶨ��Ʒ��ZnSO4��7H2O�ĺ�����ȡag��Ʒ����ˮ����������Һ����������̼������Һ��������ȫ�����ˡ�ϴ�ӳ������ڽ�����������������ȫ�ֽ⣬�Ƶ�Ϊbg�����Ʒ��ZnSO4��7H2O������������__________________________��
���𰸡�
��1����NH4��2SO4 ��2�֣�
��2����������1�֣� 3��2~5��5��1�֣�
��3��MnSO4+��NH4��2S2O8+2H2O![]() MnO2��+��NH4��2SO4+2H2SO4��3�֣�
MnO2��+��NH4��2SO4+2H2SO4��3�֣�
��4��Zn�ۣ�1�֣�
��5������Ũ����2�֣� ��ȴ�ᾧ��2�֣�
��6����100%��3�֣�
��������
���������
��1�����ڡ����̡����������ӵ�����NH4��2S2O8��������õ������ᣬ��Y�ǣ�NH4��2SO4��
��2����������ʱ��Ӧ�ȼ�����������Fe2+����ΪFe3+��������Һ��pHӦ������3��2~5��5��ʹ��ȫ������������
��3����MnO������ʱ����MnSO4��������ʱ����Ӧ����ʽ�ǣ�
MnSO4+��NH4��2S2O8+2H2O =MnO2��+��NH4��2 SO4+2H2SO4��
��4����ȥCu��Cd��Pb��ͬʱ���Ի���һЩ�ؽ���������Cu2+��Cd2+��Pb2+ת��ΪCu��Cd��Pb���ʣ��ʼ���Zn�ۡ�
��5������Ũ������ȴ�ᾧ
��6���������غ㣺ZnSO4��7H2O~ZnO���г�287��81=x��b��x=������Ʒ��ZnSO4��7H2O��������������100%��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д� ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д�����Ŀ������ʩ������Schlesinger�������������NaBH4��ˮ��Ӧ��ȡ������BH4�� + 2H2O == BO2�� + 4H2������Ӧʵ��Ϊˮ���������H+����ԭ�����о��������÷�Ӧ����H2���������������Ӱ�죬�±�ΪpH���¶ȶ�NaBH4��˥�ڵ�Ӱ�죨��˥����ָ��Ӧ�����У�ij���ʵ�Ũ�Ƚ��͵���ʼŨ��һ��ʱ�����ʱ�䣩��
��ϵ pH | ��ͬ�¶��µİ�˥�ڣ�min�� | |||
0�� | 25�� | 50�� | 75�� | |
8 | 4.32��100 | 6.19��10��1 | 8.64��10��2 | 1.22��10��2 |
10 | 4.32��102 | 6.19��101 | 8.64��100 | 1.22��100 |
12 | 4.32��104 | 6.19��103 | 8.64��102 | 1.22��102 |
14 | 4.32��106 | 6.19��105 | 8.64��104 | 1.22��104 |
��1����֪NaBH4��ˮ��Ӧ��������Һ�Լ��ԣ������ӷ���ʽ��ʾ����Һ�Լ��Ե�ԭ�� ����Һ�и�����Ũ�ȴ�С��ϵΪ ��
��2�����ϱ���֪���¶ȶ�NaBH4��ˮ��Ӧ���ʲ���������Ӱ�죿 ��
��3����Ӧ��ϵ��pHΪ�λ��NaBH4��ˮ��Ӧ�ķ�Ӧ���ʲ���Ӱ�죿 ��
�����£�N2H4���ֳ���������������һ����ɫ��״Һ�壬�е�Ϊ113.5�����º������ڲ�ͬ�¶Ⱥʹ������������ɲ�ͬ�����ͼ����

�¶Ƚϵ�ʱ��Ҫ��Ӧ����N2H4 + O2 ![]() N2 + 2H2O
N2 + 2H2O
�¶Ƚϸ�ʱ��Ҫ��Ӧ����N2H4 + 2O2 ![]() 2NO + 2H2O
2NO + 2H2O
������������Ӧ�����������գ�
��4������Ӧ����250��ʱ��ƽ�ⳣ��ΪK1��350��ʱ��ƽ������ΪK2����K1 K2�����������������������
��5����Ӧ��1100��ʱ�ﵽƽ������д�ʩ��ʹ������![]() ������� ������ĸ��ţ���
������� ������ĸ��ţ���
A�����������£�����He��
B�������������
C�����������£�����N2H4
D��ʹ�ô���
��6������n mol�º�2n molO2����ij�ݻ�Ϊn L�ĸ��������У���800����һ��ѹǿ�����ʴ����������£���Ӧ������ͬʱ�ﵽƽ�⣬ʵ����N2�IJ���x��NO�IJ���Ϊy����������·�Ӧ����ƽ�ⳣ��K= ����x��y�Ĵ���ʽ��ʾ�����ػ���