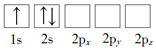
��Ŀ����
����Ŀ����.������������ֶ�����Ԫ����ɣ�����X���ɲ���ʹʪ��ĺ�ɫʯ����ֽ����������Y��YΪ�����ȡ![]() ���ú�
���ú�![]() ��������ȫ�ܽ����ҺA������ҺA�ֳ�
��������ȫ�ܽ����ҺA������ҺA�ֳ�![]() ��
��![]() ���ȷݣ��������ʵ��(��ɫ����C������
���ȷݣ��������ʵ��(��ɫ����C������![]() ��Һ)��
��Һ)��

��ش�
(1)���X������Ԫ����N��H��_______(��Ԫ�ط���)��X�Ļ�ѧʽ��_________��
(2)��ҺBͨ�����![]() �õ���ɫ����C�����ӷ���ʽ��______________��
�õ���ɫ����C�����ӷ���ʽ��______________��
(3)д��һ�����Ϸ�Ӧ(�û�ѧ����ʽ�����ӷ���ʽ��ʾ)_____________��Ҫ��ͬʱ���㣺
������һ�ַ�Ӧ������Ԫ�ر�����X�г�N��H�������Ԫ�أ�
�ڷ�Ӧԭ������![]() ����ͬ��
����ͬ��
��.ij��ȤС��Ϊ��֤Ũ��������ʽ���ʵ�飬��ͼ��ʵ���й۲쵽�������У���ƿ���а������ձ��г��ְ�ɫ��������ش�

(1)��Ũ�����Ũ�����Ͽɲ���![]() �����ԭ����________��
�����ԭ����________��
(2)�ձ��г��ְ�ɫ������ԭ����_____________��
���𰸡�![]()
![]()
![]()
![]()
![]() ��
��![]() ����Ũ�����е�ˮ���ҷ��ȵ���
����Ũ�����е�ˮ���ҷ��ȵ���![]() �ӷ�
�ӷ� ![]() ����Ὣ
����Ὣ![]() ��������
��������![]() ��������
��������![]()
��������
������ɿ�֪������X�ɲ�����ʹʪ��ĺ�ɫʯ����ֽ�����Ĵ���������Y����YΪNH3����ʵ������ͼ�з�����֪�����B��ͨ�������CO2����������NaOH��Һ�İ�ɫ����C����CΪAl(OH)3����DΪAl2O3��E��AgCl�����ͼ����������ԭ���غ㣬���Լ��������Ԫ�ص����ʵ��������X�Ļ�ѧʽ���ٸ����������ʽ��н��
��.��1���ɷ�����֪��YΪNH3����ʵ������ͼ�з�����֪�����B��ͨ�������CO2����������NaOH��Һ�İ�ɫ����C����CΪAl(OH)3����DΪAl2O3��E��AgCl������ԭ���غ��֪��A1��Һ�к���Nԭ�ӵ����ʵ���Ϊ��![]() ��Alԭ�ӵ����ʵ���Ϊ��
��Alԭ�ӵ����ʵ���Ϊ��![]() ��A2��Һ�к��е�Cl-�����ʵ���Ϊ��
��A2��Һ�к��е�Cl-�����ʵ���Ϊ��![]() ��
��
��һ����Һ�к��е�Hԭ�ӵ����ʵ���Ϊ�� ����X�к�������Ԫ�ؼ�N��H��Al��Cl���������Ϊ��
����X�к�������Ԫ�ؼ�N��H��Al��Cl���������Ϊ��![]() ����X�Ļ�ѧʽΪ��AlCl3NH3���ʴ�Ϊ��Al Cl AlCl3 NH3��
����X�Ļ�ѧʽΪ��AlCl3NH3���ʴ�Ϊ��Al Cl AlCl3 NH3��
(2)���ݷ�����1����֪����ҺB��ͨ�������CO2�����������ӷ���ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3)�������������������ٷ���������X(AlCl3NH3)��NH3��AlCl3ͨ����λ����ϳɵĻ���������뵽������NH3��H2O��Ӧ���ʿ��Ժܿ�ó��÷�Ӧ�����ӷ���ʽΪAlCl3+Cl-=![]() /span>����AlCl3+NH3=AlCl3NH3���ʴ�Ϊ��AlCl3+Cl-=
/span>����AlCl3+NH3=AlCl3NH3���ʴ�Ϊ��AlCl3+Cl-=![]() ����AlCl3+NH3=AlCl3NH3��
����AlCl3+NH3=AlCl3NH3��
��.(1)����Ũ���������ˮ����Ũ����ϡ���Ǹ����ȹ��̣�����HCl�Ļӷ�����Ũ��������������¶����߶����Ӷ��ó���Ũ�����Ũ�������Ʊ�HCl��ԭ����Ũ��������Ũ�����е�ˮ���ҷ��ȣ�ʹŨ����Ļӷ�����ǿ��ʹHCl�ӷ��������ʴ�Ϊ��Ũ��������Ũ�����е�ˮ���ҷ��ȣ�ʹŨ����Ļӷ�����ǿ��ʹHCl�ӷ�������
(2)Ũ������Ȼ�ѻӷ�����Ҳ����HCl����������������H2SO4���ӣ���Ba(NO3)2��Ӧ�������ᱵ��ɫ�������ʴ�Ϊ��HCl�����ܹ�����H2SO4���ӣ���Ba(NO3)2��Ӧ����BaSO4������

����Ŀ��ijѧϰС�鰴����ʵ������̽�������е⺬���IJⶨ�͵����ȡ��

��ʵ��һ���⺬���IJⶨ��ȡ0.0100mol/L��AgNO3����Һװ��ζ��ܣ�ȡ100.00mL������ȡԭҺ���ζ��أ��õ��Ƶζ����ⶨ�⺬������õĵ綯��(E)��ӳ��Һ��c(I-)�ı仯�������������±���
V(AgNO3)/mL | 15.00 | 19.00 | 19.80 | 19.98 | 20.00 | 20.02 | 21.00 | 23.00 | 25.00 |
E/mV | -225 | -200 | -150 | -100 | 50.0 | 175 | 275 | 300 | 325 |
�ش��������⣺
(1)ʵ����������������__(����������)����ɵġ�
(2)�����־������������������̣�__
(3)���ݱ��������жϵζ��յ�ʱ��ȥAgNO3��Һ�����Ϊ__mL������ú����е�������ٷֺ���Ϊ__����
��ʵ����������ȡ�����ƺ�����ȡԭҺ���ס�������ʵ�鷽�����£�

��֪��3I2+6NaOH=5NaI+NaIO3+3H2O��
(4)������O2�����ܴ���O2��������ʶ�Ӧ�ĵ���ʽΪ__
(5)��Ҫ�ⶨ��I2+ˮ��Һ���е�ĺ���������ѡ��__��ָʾ������Na2S2O3��Һ�ζ����ζ��յ��������__��
(6)���õ���I2+ˮ��Һ��ʱ����������Һ���࣬��Na2S2O3��Һ�ζ�ʱ��������Ե�������������ԭ��Ϊ__(�����ӷ���ʽ��ʾ)��
����Ŀ����1����������Ϊ���������ں��º����ܱ������г���һ������NO��NH3����һ�������·�����Ӧ��6NO(g) + 4NH3(g) ![]() 5N2(g) +6H2O(g)��
5N2(g) +6H2O(g)��
����˵���÷�Ӧ�Ѵﵽƽ��״̬�ı�־��_______
a����Ӧ����![]() ( NH3) =
( NH3) =![]() ( N2)
( N2)
b��������ѹǿ������ʱ��������仯
c��������N2�����ʵ�������������ʱ��������仯
d��������n(NO)��n(NH3)��n(N2)��n(H2O) = 6��4��5��6
��ij��ʵ���в��������NO��N2�����ʵ�����ʱ��仯��ͼ��ʾ��ͼ��b���Ӧ�����ʹ�ϵ��![]() (��)____
(��)____![]() (��)�����������������������d���Ӧ�����ʹ�ϵ��
(��)�����������������������d���Ӧ�����ʹ�ϵ��![]() (��)____
(��)____![]() (��)�����������
(��)�����������

��2��298 Kʱ������֪���ɱ�״����2.24 L NH3ʱ�ų�����Ϊ4.62 kJ��д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ_______���ڸ��¶��£�ȡ1 mol N2��3 mol H2����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų���������С��92.4 kJ����ԭ����________��
��3��һ�������£���2 L�ܱ������ڣ���Ӧ2NO2(g)![]() N2O4(g) ��H����180 kJ��mol��1��n(NO2)��ʱ��仯���±���
N2O4(g) ��H����180 kJ��mol��1��n(NO2)��ʱ��仯���±���
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO2)/mol | 0.040 | 0.020 | 0.010 | 0.005 | 0.005 | 0.005 |