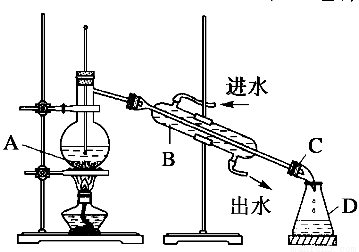
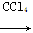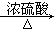ΧβΡΩΡΎ»ί
ΚΘΥ°’ΦΒΊ«ρΉή¥ΔΥ°ΝΩΒΡ97%ΓΘ»τΑ―ΚΘΥ°Β≠Μ·ΚΆΜ·ΙΛ…ζ≤ζΫαΚœΤπά¥Θ§Φ»Ω…“‘ΫβΨωΒ≠Υ°Ή ‘¥»±ΖΠΒΡΈ ΧβΘ§”÷Ω…“‘≥δΖ÷άϊ”ΟΚΘ―σΉ ‘¥ΓΘ
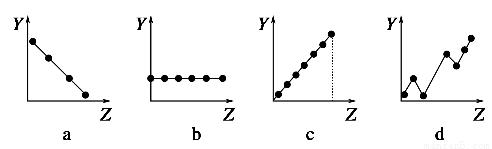
Θ®1Θ©ΙΛ“Β…œάϊ”ΟΒγΫβ±ΞΚΆ ≥―ΈΥ°Ω…÷ΤΒΟ÷Ί“ΣΜ·ΙΛ≤ζΤΖΘ§”÷≥ΤΈΣΓΑ¬»ΦνΙΛ“ΒΓ±ΓΘ“≤Ω…“‘ΒγΫβ»έ»Ύ¬»Μ·ΡΤ÷ΤΒΟΡΤΚΆ¬»ΤχΘ§ΗΟΖ¥”ΠΖΫ≥Χ ΫΈΣ________________________ΓΘ
Θ®2Θ©ΫϋΡξά¥”–»ΥΧα≥ωΝΥ“Μ÷÷άϊ”Ο¬»ΦνΙΛ“Β≤ζΤΖΦΑ¬»Μ·ΡΤ―≠ΜΖ÷ΈάμΚ§Εΰ―θΜ·ΝρΖœΤχ≤ΔΜΊ ’Εΰ―θΜ·ΝρΒΡΖΫΖ®Θ§ΗΟΖΫΖ®ΒΡΝς≥Χ»γœ¬ΘΚ
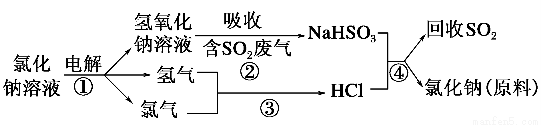
‘–¥≥ωΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΎ__________Θ§Δέ__________Θ§Δή__________ΓΘ
Θ®1Θ©2NaClΘ®»έ»ΎΘ©  2NaΘΪCl2Γϋ
2NaΘΪCl2Γϋ
Θ®2Θ©ΔΎNaOHΘΪSO2=NaHSO3
ΔέH2ΘΪCl2 2HCl
2HCl
ΔήNaHSO3ΘΪHCl=NaClΘΪSO2ΓϋΘΪH2O
ΓΨΫβΈωΓΩΘ®1Θ©ΒγΫβ»έ»ΎNaClΒΡΖΫ≥Χ ΫΈΣ2NaClΘ®»έ»ΎΘ©  2NaΘΪCl2ΓϋΓΘ
2NaΘΪCl2ΓϋΓΘ
Θ®2Θ©ΗΟΝς≥ΧΫœ«εΈζΒΊ’Ι ΨΝΥ¬»Φν≤ζΤΖΦΑNaCl―≠ΜΖ÷ΈάμΚ§SO2ΒΡΖœΤχ≤ΔΜΊ ’SO2ΒΡ‘≠άμΘ§ΗυΨί“Σ«σ–¥≥ωΖΫ≥Χ ΫΦ¥Ω…ΓΘ

ΝΖœΑ≤αœΒΝ–¥πΑΗ
 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ