��Ŀ����
11��ij�о�С��Ϊ��̽��һ����������X����������Ԫ�أ�����ɺ����ʣ���Ʋ��������ʵ�飺ʵ��һ�����Բⶨ������ͼ1װ�òⶨ����X�е�Ԫ��
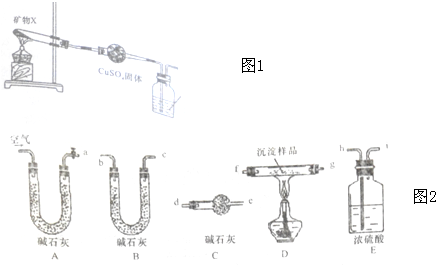
��1������һ��ʱ���۲쵽����CuSO4�����ɰ�ɫ��Ϊ��ɫ������ʯ��ˮ����ǣ�˵��X�ֽ�������CO2��H2O��
��2��ȡ�ֽ��Ĺ�������������ϡ���������ܽ�õ���ɫ��Һ��
��������ʵ֤��X�е�����Ԫ�طֱ���Cu��H��C��O��
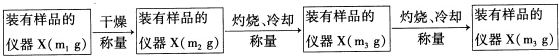
ʵ����������ⶨ��ͨ��ͼ2��ʾװ�õ����ӣ����ж����������ⶨ����ɣ�
��3��ʵ�鿪ʼʱҪͨ������Ŀ��������������ž�������CO2��ˮ������
��4����װ�õĽӿ�˳���ǣ�a��f��g��h��i��b��c��d��
��5��װ��C�������������Ƿ�ֹ�����е�CO2��ˮ�������룬Ӱ��ⶨ�����
��6��ʵ������г�ȡ����X������Ϊ11.1�ˣ���Ӧ������װ��B����������2.2�ˣ�Eװ������0.9�ˣ������X�Ļ�ѧʽΪ��Cu2��OH��2CO3��
���� I����1����ˮ����ͭ����˵����ˮ������ʯ��ˮ����ǣ�˵���ж�����̼��
��2���ֽ��Ĺ�������������ϡ���������ܽ�õ���ɫ��Һ��˵�����Ȼ�ͭ��Һ���ɣ���ϣ�1���ж�Ԫ����ɣ�
II���ѿ�����ͨ����ʯ�ң����������еĶ�����̼��ˮ������Ȼ��ͨ������װ�ã����ž�װ����ԭ�еĿ������ڼ�����������Ʒ�ֽ⣬���ɵĻ�����壬ͨ��Ũ��������ˮ����ͨ��B��ʯ�����ն�����̼���������C���Է�ֹ�����е�ˮ�����Ͷ�����̼����B�в������ţ�
��3����ͨ���������ų�װ����ԭ�еĿ�����
��4������ʵ����̷����ж���������˳��
��5�������еĶ�����̼��ˮ��������B�л�ʹ��õĶ�����̼������ƫ��
��6����Ӧ������װ��B����������2.2�ˣ������ɵĶ�����̼Ϊ2.2g��Eװ������0.9�ˣ�������ˮΪ0.9g������Ԫ���غ����C��H��O��Cu�����ʵ������ݴ�ȷ����ѧʽ��
��� �⣺I����1����ˮ����ͭ����˵����ˮ������ʯ��ˮ����ǣ�˵���ж�����̼������X�ֽ�������CO2�ͣ�
�ʴ�Ϊ��CO2��H2O��
��2���ɣ�1��������֪X�ֽ����ɶ�����̼��ˮ����X�к���C��H��OԪ�أ��ֽ��Ĺ�������������ϡ���������ܽ�õ���ɫ��Һ��˵�����Ȼ�ͭ��Һ���ɣ���X�л�����CuԪ�أ�����X��Ԫ�����ΪCu��H��C��O��
�ʴ�Ϊ��Cu��H��C��O��
II���ѿ�����ͨ����ʯ�ң����������еĶ�����̼��ˮ������Ȼ��ͨ������װ�ã����ž�װ����ԭ�еĿ������ڼ�����������Ʒ�ֽ⣬���ɵĻ�����壬ͨ��Ũ��������ˮ����ͨ��B��ʯ�����ն�����̼���������C���Է�ֹ�����е�ˮ�����Ͷ�����̼����B�в������ţ�
��3��װ�����п��������еĶ�����̼��ˮ������ʵ���и��ţ�����ʵ�鿪ʼʱҪͨ������Ŀ������ž�������CO2��ˮ������
�ʴ�Ϊ���ž�������CO2��ˮ������
��4���ɷ�����֪װ�õ�����˳��Ϊ��a��f��g��h��i��b��c��
�ʴ�Ϊ��f��g��h��i��b��c��
��5�������еĶ�����̼��ˮ��������B�л�ʹ��õĶ�����̼������ƫ������Bװ�ú�������һ��ʢ�м�ʯ�ҵĸ����C����ֹ�����е�CO2��ˮ�������룬Ӱ��ⶨ�����
�ʴ�Ϊ����ֹ�����е�CO2��ˮ�������룬Ӱ��ⶨ�����
��6����Ӧ������װ��B����������2.2�ˣ������ɵĶ�����̼Ϊ2.2g����n��CO2��=n��C��=$\frac{2.2g}{44g/mol}$=0.05mol��
Eװ������0.9�ˣ�������ˮΪ0.9g����n��H��=2n��H2O��=$\frac{0.9g}{18g/mol}$��2=0.1mol��
�ɶ��Է�����֪���ֽ��Ĺ�������������ϡ���������ܽ�õ���ɫ��Һ����ֽ��Ĺ���ΪCuO����CuO������Ϊ11.1g-2.2g-0.9g=8.0g��
����n��CuO��=$\frac{8.0g}{80g/mol}$=0.1mol��
��Ʒ��OԪ�ص����ʵ�����n��O��=0.05��2+0.05+0.1=0.25mol��
����Ʒ��N��Cu����N��H����N��C����N��O��=0.1mol��0.05mol��0.1mol��0.25mol=2��1��2��5�����仯ѧʽΪCu2��OH��2CO3��
�ʴ�Ϊ��Cu2��OH��2CO3��
���� ���⿼�������ʻ�ѧʽ��ȷ�����漰���ʵļ��顢���ʵ����ʡ�ԭ���غ��ڼ����е�Ӧ�õ�֪ʶ�㣬��ϤԪ�ػ�����������ǽⱾ��ؼ�������ʵ��ԭ���Լ�ʵ��װ�õ�����˳���ÿһ�������ķ�Ӧ����Ӧ�����ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �õ����ͨ��״��ĵ缫Ϊ��Դ������ | |
| B�� | ��������ԭ | |
| C�� | ��������ͨ��4mol����ʱ�����ļ״������ʵ���Ϊ1mol | |
| D�� | ��Һ�е�H+��ͨ�״��ĵ缫�˶� |
| A�� | C2H6��C4H10 | B�� | C3H8��C5H10 | C�� | C6H6��C6H12 | D�� | CH4��C2H2 |
| A�� | ����Ԫ�طֲ��ڶ����ںͳ������������Ԫ�ؽ��ֲ��ڳ������� | |
| B�� | Ԫ�����ڱ��У�λ��ͬһ�����Ԫ�ص�ԭ��������������ͬ���ҵ��������� | |
| C�� | Ԫ�����ڱ��У����ڵ��������Ǹ�����Ԫ��ԭ�Ӿ��еĵ��Ӳ��� | |
| D�� | Ԫ�����ڱ���7�����У�7�����ڣ�18�����У�18���� |
| A�� | 2 mL 0.5 mol/L��������Һ�е����������ƵõĹ��ὺ���У���������ĿΪ0.001NA | |
| B�� | 8.4 g NaHCO3�����к��е���������Ϊ0.3NA | |
| C�� | 25��ʱ��1 L pH=l��CH3COOH��Һ�к���H+��ĿΪ0��lNA | |
| D�� | 7.8 g Na2O2�����к��еĹ��õ��Ӷ���ĿΪ0.2NA |
 ������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȣ������жϲ���ȷ���ǣ�������
������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȣ������жϲ���ȷ���ǣ�������| A�� | �����̬�⻯������ȶ��ԣ�R��Q | |
| B�� | ����������Ӧˮ��������ԣ�Q��W | |
| C�� | ԭ�Ӱ뾶��T��Q��R | |
| D�� | T������������Ӧˮ������Һһ���Լ��� |
| A�� | �������ڢ�A�� | B�� | �ڶ����ڢ�A�� | C�� | �������ڢ�A�� | D�� | �ڶ����ڢ�A�� |