��Ŀ����
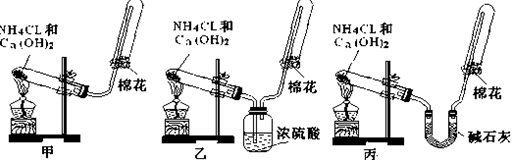
����װ�ö�������ʵ������ȡ����������գ����ɹ�ѡ�õ��Լ�Ϊ����NaOH��Һ ��NaOH���� ��������ʯ�� ��NH4Cl��Һ ��Ũ��ˮ ����ʯ�� ����ˮCaCl2��
��1������Aװ�ã�����ȡ�����Ļ�ѧ����ʽ��
��2������Bװ����ȡ��������ѡ�õ�ҩƷ��
��3������Cװ����ȡ����������ȡ�����Ļ�ѧ����ʽ��
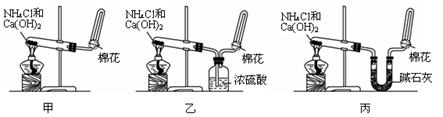
��������1��Aװ�����ڹ̹̻�ϼ���װ�ã��������Ȼ�狀���ʯ����ȡ�������������ڼ������壬�����ü��Ի��������ʸ�����������Ȼ��Ƹ��������ʹʪ��ĺ�ɫʯ����ֽ����ɫ��
��2��Bװ�����ڹ�Һ��ϲ�������װ�ã�������Ũ��ˮ����ʯ�ҡ��������ƹ�����ȡ������
��3����װ�����ڹ�Һ��ϼ�����װ�ã���������κ�ǿ�Ӧ��ȡ����������Ҳ�����ø�װ����ȡ��
��2��Bװ�����ڹ�Һ��ϲ�������װ�ã�������Ũ��ˮ����ʯ�ҡ��������ƹ�����ȡ������
��3����װ�����ڹ�Һ��ϼ�����װ�ã���������κ�ǿ�Ӧ��ȡ����������Ҳ�����ø�װ����ȡ��
����⣺��1��Aװ�����ڹ̹̻�ϼ���װ�ã��������Ȼ�狀���ʯ����ȡ��������Ӧ����ʽΪ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
�������ڼ������壬�����ü��Ի��������ʸ�����������Ȼ��Ƹ�����������������ƹ����������ʯ�Ҹ��ﰱ����
������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����Կ�����ʪ��ĺ�ɫʯ����ֽ���鰱��������鷽���ǣ�ʪ��ĺ�ɫʯ����ֽ�ӽ��Թܿڣ���ֽ����ɫ��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O���ڢۣ�ʪ��ĺ�ɫʯ����ֽ�ӽ��Թܿڣ���ֽ����ɫ��
��2��Bװ�����ڹ�Һ��ϲ�������װ�ã�һˮ�ϰ��ֽ⣬�����¶ȴٽ�һˮ�ϰ��ֽ⣬��ʯ�Һ�ˮ��Ӧ�ų������ȣ��������ƹ����ܽ�ʱ�ų������ȣ����Կ�����Ũ��ˮ����ʯ�ҡ��������ƹ�����ȡ��������ѡ�ݢۻ�ݢڣ�
��3����װ�����ڹ�Һ��ϼ�����װ�ã���������κ�ǿ�Ӧ��ȡ�������磺�ڼ��������£����Ȼ�狀�����������Һ��ȡ��������Ӧ����ʽΪ��NH4Cl+NaOH
NaCl+NH3��+H2O���ڼ��������£���Ũ����Ͷ���������ȡ��������������Ҳ�����ø�װ����ȡ��
�ʴ�Ϊ��NH4Cl+NaOH
NaCl+NH3��+H2O��������
| ||
�������ڼ������壬�����ü��Ի��������ʸ�����������Ȼ��Ƹ�����������������ƹ����������ʯ�Ҹ��ﰱ����
������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����Կ�����ʪ��ĺ�ɫʯ����ֽ���鰱��������鷽���ǣ�ʪ��ĺ�ɫʯ����ֽ�ӽ��Թܿڣ���ֽ����ɫ��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
| ||
��2��Bװ�����ڹ�Һ��ϲ�������װ�ã�һˮ�ϰ��ֽ⣬�����¶ȴٽ�һˮ�ϰ��ֽ⣬��ʯ�Һ�ˮ��Ӧ�ų������ȣ��������ƹ����ܽ�ʱ�ų������ȣ����Կ�����Ũ��ˮ����ʯ�ҡ��������ƹ�����ȡ��������ѡ�ݢۻ�ݢڣ�
��3����װ�����ڹ�Һ��ϼ�����װ�ã���������κ�ǿ�Ӧ��ȡ�������磺�ڼ��������£����Ȼ�狀�����������Һ��ȡ��������Ӧ����ʽΪ��NH4Cl+NaOH
| ||
�ʴ�Ϊ��NH4Cl+NaOH
| ||
���������⿼���˰�����ʵ�����Ʒ������ݷ�Ӧ���״̬����Ӧ����ȷ����Ӧװ�ã������ķ�Ӧװ���У��̹̼����͡���Һ�������͡���Һ�����͡�ҺҺ�����ͣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ

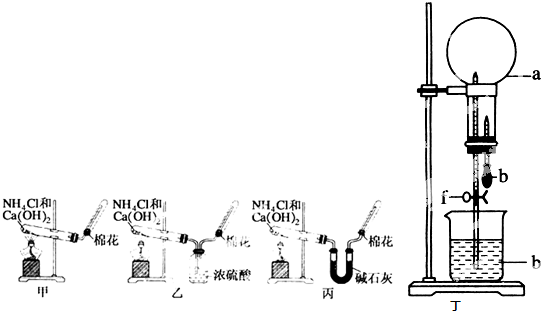

 B��������������ˮ
B��������������ˮ