ΧβΡΩΡΎ»ί
Ρ≥Ά§―ßΙΚ¬ρΝΥ“ΜΤΩΓΝΓΝ≈ΤΓΑ84œϊΕΨ“ΚΓ±Θ§≤ι‘ΡœύΙΊΉ ΝœΚΆœϊΕΨ“ΚΑϋΉΑΥΒΟςΒΟΒΫ»γœ¬–≈œΔΘΚ
ΓΑ84œϊΕΨ“ΚΓ±ΘΚΚ§25%NaClO 1 000 mLΓΔΟήΕ»1.19 gΓΛcmΘ≠3Θ§œΓ Ά100±Ε(ΧεΜΐ±»)Κσ Ι”ΟΓΘ
«κΗυΨί“‘…œ–≈œΔΚΆœύΙΊ÷Σ ΕΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)ΗΟΓΑ84œϊΕΨ“ΚΓ±ΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ________ molΓΛLΘ≠1ΓΘ
(2)ΗΟΆ§―ß»Γ100 mLΗΟΓΑ84œϊΕΨ“ΚΓ±œΓ ΆΚσ”Ο”ΎœϊΕΨΘ§œΓ ΆΚσΒΡ»ή“Κ÷–c(NaΘΪ)ΘΫ________ molΓΛLΘ≠1(ΦΌ…ηœΓ ΆΚσ»ή“ΚΟήΕ»ΈΣ1.0 gΓΛcmΘ≠3)ΓΘ
(3)Ρ≥ Β―ι–η”Ο480 mLΚ§25%NaClOΒΡœϊΕΨ“ΚΓΘΗΟΆ§―ß≤Έ‘ΡΗΟΓΑ84œϊΕΨ“ΚΓ±ΒΡ≈δΖΫΘ§”ϊ”ΟNaClOΙΧΧε≈δ÷ΤΗΟœϊΕΨ“ΚΓΘ
ΔΌœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «________ΓΘ

AΘ°»γ…œΆΦΥυ ΨΒΡ“«Τς÷–Θ§”–ΥΡ÷÷ «≤Μ–η“ΣΒΡΘ§ΜΙ–η“Μ÷÷≤ΘΝß“«Τς
BΘ°»ίΝΩΤΩ”Ο’τΝσΥ°œ¥ΨΜΚσΘ§”ΠΚφΗ…≤≈Ρή”Ο”Ύ»ή“Κ≈δ÷Τ
CΘ°άϊ”ΟΙΚ¬ρΒΡ…ΧΤΖNaClOά¥≈δ÷ΤΩ…ΡήΒΦ÷¬ΫαΙϊΤΪΒΆ
DΘ°–η“Σ≥ΤΝΩΒΡNaClOΙΧΧε÷ ΝΩΈΣ143 g
ΔΎ‘Ύ≈δ÷ΤΙΐ≥Χ÷–Θ§œ¬Ν–≤ΌΉςΩ…Ρή Ι≈δ÷ΤΒΡ»ή“ΚΒΡ≈®Ε»ΤΪ¥σΒΡ «________ΓΘ
AΘ°…’±≠÷–»ή“ΚΉΣ“ΤΒΫ»ίΝΩΤΩ÷– ±Θ§Έ¥œ¥Β”…’±≠
BΘ°Ε®»ί ±Θ§Η© ”ΩΧΕ»œΏ
CΘ°Ε®»ί ±Θ§―ω ”ΩΧΕ»œΏ
DΘ°“Τ“Κ ±Θ§”–…ΌΝΩ“ΚΧεΫΠ≥ω
ΓΓ(1)4.0ΓΓ(2)0.04ΓΓ(3)ΔΌCΓΓΔΎB
ΓΨΫβΈωΓΩΓΓ(1)ΗυΨίcΘΫ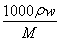 Θ§c(NaClO)ΘΫ4Θ°0 molΓΛLΘ≠1ΓΘ
Θ§c(NaClO)ΘΫ4Θ°0 molΓΛLΘ≠1ΓΘ
(2)œΓ Ά100±ΕΚσ≈®Ε»ΈΣ‘≠ά¥ΒΡ1%ΓΘ
(3)ΔΌ―ΓœνAΘ§–η”ΟΆ–≈ΧΧλΤΫ≥ΤΝΩNaClOΙΧΧεΘ§–η”Ο…’±≠ά¥»ήΫβNaClOΘ§–η”Ο≤ΘΝßΑτΫχ––ΫΝΑηΚΆ“ΐΝςΘ§–η”Ο»ίΝΩΤΩΚΆΫΚΆΖΒΈΙήά¥Ε®»ίΘ§ΆΦ Ψ÷–ΒΡ…œΟφΥΡ÷÷“«Τς≤Μ–η“ΣΘ§ΒΪΜΙ–η≤ΘΝßΑτΚΆΫΚΆΖΒΈΙήΓΘ―ΓœνBΘ§≈δ÷ΤΙΐ≥Χ÷––η“ΣΦ”»κΥ°Θ§Υυ“‘Ψ≠œ¥Β”Η…ΨΜΒΡ»ίΝΩΤΩ≤Μ±ΊΚφΗ…Κσ‘Ό Ι”ΟΓΘ―ΓœνCΘ§”…”ΎNaClO“ΉΈϋ ’Ω’Τχ÷–ΒΡH2OΓΔCO2Εχ±δ÷ Θ§Υυ“‘…ΧΤΖNaClOΩ…Ρή≤ΩΖ÷±δ÷ ΒΦ÷¬NaClOΦθ…ΌΘ§≈δ÷ΤΒΡ»ή“Κ÷–»ή÷ ΒΡΈο÷ ΒΡΝΩΦθ–ΓΘ§ΫαΙϊΤΪΒΆΓΘ―ΓœνDΘ§”Π―Γ»Γ500 mLΒΡ»ίΝΩΤΩΫχ––≈δ÷ΤΘ§»ΜΚσ»Γ≥ω480 mLΦ¥Ω…Θ§Υυ“‘–η“ΣNaClOΒΡ÷ ΝΩΘΚ0.5 LΓΝ4.0 molΓΛLΘ≠1ΓΝ74.5 gΓΛmolΘ≠1ΘΫ149 gΓΘ
ΔΎ”…cΘΫ ≈–ΕœΘΚAΓΔD―Γœν÷– ΙnΤΪ–ΓΘ§≈®Ε»ΤΪ–ΓΘΜB―Γœν÷–Η© ”ΩΧΕ»œΏΘ§ ΙVΤΪ–ΓΘ§≈®Ε»ΤΪ¥σΘΜC―Γœν÷–―ω ”ΩΧΕ»œΏΘ§ ΙVΤΪ¥σΘ§≈®Ε»ΤΪ–ΓΓΘ
≈–ΕœΘΚAΓΔD―Γœν÷– ΙnΤΪ–ΓΘ§≈®Ε»ΤΪ–ΓΘΜB―Γœν÷–Η© ”ΩΧΕ»œΏΘ§ ΙVΤΪ–ΓΘ§≈®Ε»ΤΪ¥σΘΜC―Γœν÷–―ω ”ΩΧΕ»œΏΘ§ ΙVΤΪ¥σΘ§≈®Ε»ΤΪ–ΓΓΘ

 ΦΤΥψΗΏ ÷œΒΝ–¥πΑΗ
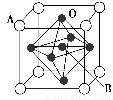
ΦΤΥψΗΏ ÷œΒΝ–¥πΑΗ2012Ρξ12‘¬Θ§Έ“Ιζ≥…ΙΠ Βœ÷ΦΏΓΣ15Ζ…Μζ‘ΎΓΑΝ…ΡΰΚ≈Γ±ΚΫΡΗ…œΒΡΤπΫΒ Β―ιΘ§Ϋ®‘λΚΫΡΗΤΫΧ®ΚΆΫΔ‘ΊΖ…Μζάκ≤ΜΩΣ»γν―ΓΔΈο÷ AΒ»≤ΡΝœΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)–¥≥ων―ΜυΧ§‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΘΚ___________________________ΓΘ
(2)“―÷Σ‘ΣΥΊA¥Π”ΎΒΎ3÷ήΤΎΘ§Τδ‘≠Ή”ΒΡΒΎ“Μ÷ΝΒΎΥΡΒγάκΡή ΐΨί»γ±μΥυ ΨΘΚ
ΒγάκΡή(kJ/mol) | I1 | I2 | I3 | I4 |
A | 738 | 1 451 | 7 733 | 10 540 |
ΔΌAΒΡ‘ΣΥΊΖϊΚ≈ΈΣ________ΓΘ
ΔΎ”κ‘ΣΥΊAΆ§÷ήΤΎΒΡΒγΗΚ–‘Ήν¥σΒΡ‘ΣΥΊΈΣ________(Χν‘ΣΥΊΖϊΚ≈)Θ§ΗΟ‘ΣΥΊΡή”κΆ§÷ήΤΎΒΡΝμ“Μ÷÷Ζ«Ϋπ τ‘ΣΥΊ–Έ≥…5‘≠Ή”Ζ÷Ή”Θ§ΗΟΖ÷Ή”ΒΡ÷––Ρ‘≠Ή”≤…”ΟΒΡ‘”Μ·ΖΫ Ϋ «________Θ§ΗΟΖ÷Ή”ΒΡΩ’ΦδΙΙ–ΆΈΣ________ΓΘ
(3)ν―ΟΨΚœΫπ «÷Τ‘λΗΏ–‘ΡήΖ…ΜζΒΡ÷Ί“Σ≤ΡΝœΓΘ“―÷Σν―ΓΔΟΨΫπ τΨυ≤…”ΟΝυΖΫΉνΟήΕ―Μΐ( )Θ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «________(Χν–ρΚ≈)ΓΘ
)Θ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «________(Χν–ρΚ≈)ΓΘ
AΘ°”Ον―ΟΨΚœΫπά¥÷Τ‘λΗΏ–‘ΡήΖ…ΜζΘ§÷ς“Σ”…”ΎΤδΦέΗώΑΚΙσΘ§÷Τ‘λ≥ωΒΡΖ…ΜζΡή¬τΗωΚΟΦέ«°
BΘ°‘ΎΗΏΈ¬œ¬«–ΗνΫπ τν―≤ΜΜα≤ζ…ζ»ΈΚΈΑ≤»Ϊ ¬Ι
CΘ°ν―ΓΔΟΨΫπ τΨßΧε÷–Θ§Τδ≈δΈΜ ΐΨυΈΣ12
DΘ°Ϋπ τν―ΒΡ»έΒψΚήΗΏ(1 668 Γφ)Θ§”κΫπ τΦϋΙΊœΒ≤Μ¥σ
(4)ΗΤν―–ΆΗ¥Κœ―θΜ·Έο(»γΆΦΥυ Ψ)Ω…”Ο”Ύ÷Τ‘λΚΫΩ’ΡΗΫΔ÷–ΒΡ»»Οτ¥ΪΗ–ΤςΘ§ΗΟΨßΧε“‘A‘≠Ή”ΈΣΨßΑϊΒΡΕΞΒψΘ§AΩ…“‘ «CaΓΔSrΓΔBaΜρPbΘ§Β±B «VΓΔCrΓΔMnΜρFe ±Θ§’β÷÷Μ·ΚœΈοΨΏ”–ΚήΚΟΒΡΒγΜ·―ß–‘ΡήΓΘ”ΟAΓΔBΓΔO±μ ΨΗΤν―–ΆΗ¥Κœ―θΜ·ΈοΨßΧεΒΡΜ·―ß ΫΈΣ________ΓΘ