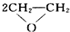��Ŀ����
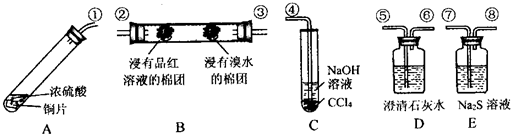
��ʮ���塱�滮�еĻ��������ǵ�2015��ĩ���ҹ�����Ҫ��Ⱦ�������̼��2010������10%��ij��ѧ��ȤС���������ͭ��Ũ������ȡSO2�������ν��м���SO2������������������ʡ���ԭ�ԡ�Ư���Ժ������Ե�ʵ�飬����������ͼ��ʾ��ͼ�����ӽ��ܡ��гֺͼ���װ�þ���ʡ�ԣ���
��ش��������⣺
��1������װ�õĽӿ�����˳�������Ǣ١�
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��
��3��֤��SO2���������Ե�������
��4��C�Թ���NaOH��Һ��������
��5��ij������ʯ��ʯ--ʯ��ʪ�����������������ͺ���ȼ��ȼ���ŷŵ�SO2��ͬʱ�����ʯ�ࣨ2CaSO4?H2O���������˻�����
�ٸù���������SO2��ʲô���ʣ�
��д���ù��յĻ�ѧ����ʽ

��ش��������⣺
��1������װ�õĽӿ�����˳�������Ǣ١�
�ݡ��ޡ��ۡ��ڡ��ߡ������
�ݡ��ޡ��ۡ��ڡ��ߡ������
����ܱ�ţ�����2��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��
Cu+2H2SO4��Ũ��
CuSO4+2SO2��+2H2O
| ||
Cu+2H2SO4��Ũ��
CuSO4+2SO2��+2H2O
��
| ||
��3��֤��SO2���������Ե�������
E�Թ�����Һ�����
E�Թ�����Һ�����
��֤��SO2���л�ԭ�Ե�ʵ���У���Ӧ�����ӷ���ʽ��SO2+Br2+2H2O=4H++SO42-+2Br-
SO2+Br2+2H2O=4H++SO42-+2Br-
����4��C�Թ���NaOH��Һ��������
����δ��Ӧ�Ķ�������������Ⱦ����
����δ��Ӧ�Ķ�������������Ⱦ����
��CCl4����������ֹ����
��ֹ����
����5��ij������ʯ��ʯ--ʯ��ʪ�����������������ͺ���ȼ��ȼ���ŷŵ�SO2��ͬʱ�����ʯ�ࣨ2CaSO4?H2O���������˻�����
�ٸù���������SO2��ʲô���ʣ�
���ԡ���ԭ��
���ԡ���ԭ��
����д���ù��յĻ�ѧ����ʽ
2CaCO3+2SO2+O2+H2O=2CaSO4?H2O+2CO2
2CaCO3+2SO2+O2+H2O=2CaSO4?H2O+2CO2
����������1��ͭ��Ũ������ȡSO2�������ν��м���SO2������������������ʡ���ԭ�ԡ�Ư���Ժ������Ե�ʵ�飬ͭ��Ũ������ȷ�Ӧ���ɶ����������壬ͨ������ʯ��ˮ�����֤���������������������ͨ��Bװ�ü����������Ļ�ԭ�Ժ�Ư���ԣ�ͨ��Eװ�ú����Ʒ�Ӧ���ɵ�����֤����������������ԣ����ͨ������������Һ�����չ����Ķ��������ֹ��Ⱦ���������������������Ȼ�̼��ͨ�����Ȼ�̼��Һ�п��Է�ֹ������
��2��ͭ��Ũ������ȷ�Ӧ��������ͭ�����������ˮ��
��3��֤������������������Ե�װ����E�������Ʒ�Ӧ���ɵ�����֤����������ԭ�Ե�װ���������嵥�ʺͶ�������Ӧ��
��5���ٶ��������̼��Ʒ�Ӧ�����������֤��������������ԣ����ɵ�������Ʊ������е���������Ϊ����ƣ������˶�������Ļ�ԭ�ԣ�
�ڶ���������̼��Ʒ�Ӧ������������������̼�����������ˮ���ڵ������±�������������CaSO4?2H2O��
��2��ͭ��Ũ������ȷ�Ӧ��������ͭ�����������ˮ��
��3��֤������������������Ե�װ����E�������Ʒ�Ӧ���ɵ�����֤����������ԭ�Ե�װ���������嵥�ʺͶ�������Ӧ��
��5���ٶ��������̼��Ʒ�Ӧ�����������֤��������������ԣ����ɵ�������Ʊ������е���������Ϊ����ƣ������˶�������Ļ�ԭ�ԣ�
�ڶ���������̼��Ʒ�Ӧ������������������̼�����������ˮ���ڵ������±�������������CaSO4?2H2O��
����⣺��1��ͭ��Ũ������ȡSO2�������ν��м���SO2������������������ʡ���ԭ�ԡ�Ư���Ժ������Ե�ʵ�飬ͭ��Ũ������ȷ�Ӧ���ɶ����������壬ͨ������ʯ��ˮ�����֤���������������������ͨ��Bװ�ü����������Ļ�ԭ�Ժ�Ư���ԣ�ͨ��Eװ�ú����Ʒ�Ӧ���ɵ�����֤����������������ԣ����ͨ������������Һ�����չ����Ķ��������ֹ��Ⱦ���������������������Ȼ�̼��ͨ�����Ȼ�̼��Һ�п��Է�ֹ����������˳��Ϊ���ݡ��ޡ��ۡ��ڡ��ߡ�����ܣ�
�ʴ�Ϊ���ݡ��ޡ��ۡ��ڡ��ߡ�����ܣ�
��2��ͭ��Ũ������ȷ�Ӧ��������ͭ�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��Cu+2H2SO4��Ũ��
CuSO4+2SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+2SO2��+2H2O��
��3��֤������������������Ե�װ����E�������Ʒ�Ӧ���ɵ�����Ӧ�Ļ�ѧ����ʽΪSO2+2S2-+2H2O=3S��+4OH-��֤����������ԭ�Ե�װ���������嵥�ʺͶ�������Ӧ����Ӧ�����ӷ���ʽΪ��SO2+Br2+2H2O=4H++SO42-+2Br-��
�ʴ�Ϊ��E�Թ�����Һ����ǣ�SO2+Br2+2H2O=4H++SO42-+2Br-
��5���ٶ��������̼��Ʒ�Ӧ�����������֤��������������ԣ����ɵ�������Ʊ������е���������Ϊ����ƣ������˶�������Ļ�ԭ�ԣ�
�ʴ�Ϊ�����ԡ���ԭ�ԣ�
�ڶ���������̼��Ʒ�Ӧ������������������̼�����������ˮ���ڵ������±�������������CaSO4?2H2O����Ӧ�Ļ�ѧ����ʽΪ��2CaCO3+2SO2+O2+H2O=2CaSO4?H2O+2CO2���ʴ�Ϊ��2CaCO3+2SO2+O2+H2O=2CaSO4?H2O+2CO2��
�ʴ�Ϊ���ݡ��ޡ��ۡ��ڡ��ߡ�����ܣ�
��2��ͭ��Ũ������ȷ�Ӧ��������ͭ�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��Cu+2H2SO4��Ũ��
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
��3��֤������������������Ե�װ����E�������Ʒ�Ӧ���ɵ�����Ӧ�Ļ�ѧ����ʽΪSO2+2S2-+2H2O=3S��+4OH-��֤����������ԭ�Ե�װ���������嵥�ʺͶ�������Ӧ����Ӧ�����ӷ���ʽΪ��SO2+Br2+2H2O=4H++SO42-+2Br-��
�ʴ�Ϊ��E�Թ�����Һ����ǣ�SO2+Br2+2H2O=4H++SO42-+2Br-
��5���ٶ��������̼��Ʒ�Ӧ�����������֤��������������ԣ����ɵ�������Ʊ������е���������Ϊ����ƣ������˶�������Ļ�ԭ�ԣ�
�ʴ�Ϊ�����ԡ���ԭ�ԣ�
�ڶ���������̼��Ʒ�Ӧ������������������̼�����������ˮ���ڵ������±�������������CaSO4?2H2O����Ӧ�Ļ�ѧ����ʽΪ��2CaCO3+2SO2+O2+H2O=2CaSO4?H2O+2CO2���ʴ�Ϊ��2CaCO3+2SO2+O2+H2O=2CaSO4?H2O+2CO2��
���������⿼�����������ʵ�ʵ����֤������ʵ��װ��ѡ���������ʺ�װ��ѡ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ